Abstract
The purpose of this article is to evaluate the relationship between the nutrition-based microenvironment and clinicopathological information for gastric cancer patients and to investigate the prognostic value of nutrition index for gastric cancer patients undergoing total gastrectomy. We retrospectively collected clinical information of 245 gastric cancer patients who underwent total gastrectomy in our hospital between January 1st 2005 and December 30th 2015. According to the prognostic nutritional index (PNI) level, they were divided into low PNI (< 43) group and high PNI (≥ 43) group. The relationship between PNI and the disease-free survival (DFS) and overall survival (OS) were analyzed by statistical analysis. Univariate analyses demonstrated that TNM stage (p = 0.025), patients age (p = 0.042), lymph node metastasis (p = 0.028), tumor differentiation (p = 0.037) and a low PNI (p = 0.033) were closely correlated with a poor prognosis. In multivariate analysis, TNM stage (p = 0.027) and a low PNI (p = 0.041) were found to be independently associated with poor survival. Additionally, when age was considered as a stratified factor, univariate analyses demonstrated that low PNI correlated with shorter DFS in non-elderly (< 65) patients (p = 0.022) and shorter DFS (p = 0.036) and OS (p = 0.047) in elderly (≥ 65) patients. The low prognostic nutritional index is an independent risk factor associated with poor gastric cancer survival which represents the nutritional microenvironment. Patients with low pre-operative prognostic nutritional index levels should be observed more closely after surgery to prevent the occurrence of post-operative complications in the near future.
Similar content being viewed by others
Introduction
Malignancy may be described as a state formed in the setting of specific tumor-host relationships at the molecular and cellular microenvironment levels1. The tumor microenvironment has many differences in physical and chemical properties from the normal internal environment of the human body. The most notable features are its low oxygen, low pH and high pressure. More and more researches indicate systemic nutrition has been found to be a crucial ingredient of the tumor microenvironment that plays remarkable roles in tumor growth, progression and metastasis2. For patients with malignant tumors, the tumor itself will consume a lot of protein, causing damage to tissue structure and organ functions; in addition, gastrointestinal obstruction caused by digestive organ tumors can lead to loss of appetite, nausea, vomiting, diarrhea which is also associated with poor nutrition and immunosuppression3,4,5.
The prognostic nutritional index (PNI) is calculated by the serum ALB (albumin) and the total number of peripheral blood lymphocytes and PNI has attracted more and more attention for its convenience and significance in clinical application. Albumin represents the nutritional condition of the human body and peripheral blood lymphocyte is an important immune index, the unbalance of albumin and lymphocyte is closely correlated with poor post-operative complications and cancer outcomes which have already demonstrated by multiple cancer types such as liver cancer6, non-small cell lung cancer7, bladder cancer4, pancreatic cancer8,9, colorectal cancer10, esophageal cancer11,12,13, ovarian cancer14, and renal cell carcinoma15. As far as we know, there is limited research on PNI in gastric cancer application.
Besides, gastric cancer is a group of heterogeneous tumors based on distinctive morphological and molecular genetic features which closely correlates with the nutritional conditions, peripheral blood cells might reflect the inflammatory and immune response of patients to malignant tumors and are critical for determining the treatment response and clinical outcomes of patients16,17.
As such, the present study aimed to evaluate the prognostic impact of PNI in patients with gastric cancer after radical gastrectomy. These results may reveal the important role of nutrition-based factors in gastric cancer after radical gastrectomy and may also help to evaluate patient prognosis.
Results
Example of histological and morphological characteristics in the gastrectomy tissues
We chose a gastric cancer patient randomly and the morphology, pathology and diagnostic markers were presented in Fig. 1. The general pathology of this case was ulcer type (Fig. 1A). There was a clear contrast between the normal gastric wall and tumor tissue (Fig. 1B). The tissue of this patient showed typical immunological markers of gastric cancer, that was, CEA, CK7 and CD20 were all positive, while CDX2 was negative (Fig. 1C). The other two very important markers for the pathological diagnosis of gastric cancer were Her-2 and p53. Gastric cancer patients with Her-2 positive should be treated with anti-Her-2 medicine, and p53 reflected tumor proliferation activity, that was, patients with high p53 had high tumor cell proliferation activity. In this case, the expression of Her-2 was negative (Fig. 1D) which suggested no need of anti-Her-2 treatment and p53 was weakly positive (Fig. 1D) which indicated normal tumor activity. Another important immunological marker of gastric cancer was the identification of mismatch repair which was shown by MLH1, MSH2, MSH6 and pMS2. The expression of MLH1, MSH2, MSH6 and pMS2 was all positive (pMMR) (Fig. 1E). So, immunotherapy was not the first choice for this patient. The results from Fig. 1 indicated that the proper and accurate pathological information was critical to clinical treatment decisions for gastric cancer.
Histological and morphological characteristics in the gastrectomy tissues. (A) The general pathology of this case was ulcer type. (B) There was a clear contrast between the normal gastric wall and tumor tissue. (C) The tissue of this patient showed typical immunological markers of gastric cancer: CEA, CK7 and CD20 were all positive and CDX2 was negative. (D) The expression of Her-2 was negative and p53 was weakly positive. (E) The expression of MLH1, MSH2, MSH6 and pMS2 was all positive (p MMR).
Correlations between the PNI and clinical characteristics
The patient characteristics are shown in Fig. 2. The PNI ranged from 33.9 to 52.4 (Supplementary Fig. 1), with a median level of 45.7 and the optimal cut-off point of the PNI was 43.15 in our research. So, the patients were divided into high PNI (PNI ≥ 43 n = 97, 39.6%) and low PNI groups (PNI < 43, n = 148, 60.4%). Correlations of clinical characteristics of the pre-operative PNI are summarized in Fig. 2. Pre-operative PNI level was associated with TNM stage (p = 0.027), tumor differentiation (p = 0.039), patients age (p = 0.042) and lymph node metastasis (p = 0.045).
Predictive values of the PNI
Univariate statistical analyses demonstrated that TNM stage (hazard ratio [HR] 4.378; 95% confidence interval [CI] 2.581–6.175; p = 0.025), patients age (HR 2.116; 95% CI 0.493–4.739; p = 0.042), lymph node metastasis (HR 2.392; 95% CI 0.469–4.315; p = 0.028), tumor differentiation (HR 3.542; 95% CI 0.764–6.320; p = 0.037) and a low PNI (HR 2.573; 95% CI 0.692–4.454; p = 0.033) were significant risk factors for a poor prognosis (Table 1). In multivariate analysis, TNM stage (HR 3.771; 95% CI 1.873–5.669; p = 0.027) and a low PNI (HR 2.351; 95% CI 1.026–3.676; p = 0.041) were found to be independently associated with poor survival.
Relationships between PNI and clinicopathological features in non-elderly patients
There was a significant correlation between PNI and lymphocyte infiltration (p = 0.034), cancer differentiation (p = 0.041), TNM stage (p = 0.026) and T stage (p = 0.046) in non-elderly patients (< 65) (Fig. 3). Univariate analysis showed that TNM stage (HR 3.223; 95% CI 1.002–5.444; p = 0.019), vascular invasion (HR 1.982; 95% CI 0.649–3.315; p = 0.041), lymph node metastasis (HR 1.794; 95% CI 0.364–3.224; p = 0.044) and low PNI (HR 2.018; 95% CI 0.357–3.679; p = 0.048) were important risk factors for poor prognosis (Table 2). In multivariate analysis, TNM stage (HR 3.116; 95% CI 1.235–4.997; p = 0.027) and low PNI (HR 2.034; 95% CI 0.337–3.731; p = 0.037) were independently related to poor survival time (Table 2). In a comparative study of PNI value and survival analysis in non-elderly patients after total gastrectomy, low PNI and short disease-free survival were statistically associated (p = 0.022), but low PNI and short overall survival time was not statistically correlated (Table 3).
Relationship between PNI and clinicopathological features in 162 non-elderly (< 65) gastric cancer patients after total gastrectomy. There was a significant correlation between PNI and lymphocyte infiltration (p = 0.034), cancer differentiation (p = 0.041), TNM stage (p = 0.026) and T stage (p = 0.046) in non-elderly patients (< 65).
Relationships between PNI and clinicopathological features in elderly patients
In elderly patients (≥ 65), there was a significant correlation between PNI and lymphocyte infiltration (p = 0.021), cancer differentiation (p = 0.045) and TNM stage (p = 0.036) (Fig. 4). Univariate analysis showed that TNM stage (HR 3.381; 95% CI 1.275–5.487; p = 0.036), tumor differentiation (HR 2.256; 95% CI 0.542–3.970; p = 0.033) , lymph node metastasis (HR 2.218; 95% CI 0.562–3.874; p = 0.041) and low PNI (HR 2.229; 95% CI 0.783–3.675; p = 0.027) were important risk factors for poor prognosis (Table 4); In multivariate analysis, TNM stage (HR 2.968; 95% CI 0.723–5.213; p = 0.032) and low PNI (HR 2.427 95% CI 0.573–4.281; p = 0.028) were independently associated with poor survival time (Table 4). In a comparative study of PNI value and survival analysis in elderly patients after total gastrectomy, there were statistical correlation between low PNI and short disease-free survival time (p = 0.036) and short overall survival time as well (p = 0.047) (Table 5).
Relationship between PNI and clinicopathological features in 83 elderly (≥ 65) gastric cancer patients after total gastrectomy. In elderly patients (≥ 65), there was a significant correlation between PNI and lymphocyte infiltration (p = 0.021), cancer differentiation (p = 0.045) and TNM stage (p = 0.036).
Statistical analysis of PNI on survival parameters
We then analyzed the pre-operative PNI values of 245 patients and divided them into PNI < 43 and PNI ≥ 43 groups. As shown in Fig. 5, in non-elderly patients (< 65), low PNI is an independent prognostic factor for a short DFS; in elderly patients (≥ 65), low PNI is an independent prognostic factor for a short DFS and OS (Fig. 6).
Predictive analysis of PNI on DFS and OS after total gastrectomy on 162 non-elderly (< 65) patients with gastric cancer. (A) The effect of PNI level on DFS of non-elderly patients (< 65), low PNI value is associated with shorter DFS and it has statistical significance; (B) the effect of PNI level on OS of non-elderly patients (< 65), low PNI value is associated with shorter OS and it has no statistical significance.
Predictive analysis of PNI on DFS and OS after total gastrectomy on 83 elderly (≥ 65) patients with gastric cancer. (A) The effect of PNI level on DFS of elderly patients (≥ 65), low PNI value is associated with short DFS and it has statistical significance; (B) the effect of PNI level on OS of elderly patients (≥ 65), low PNI value is associated with short OS and it has statistical significance.
Discussion
The tumor microenvironment plays an important role in the process of tumorigenesis. Immune and nutritional status, as parts of tumor microcirculation, will undoubtedly affect the prognosis of patients. More and more evidence show that basic nutritional status and systemic inflammation are related to the long-term prognosis of cancer patients16,17,18,19,20,21,22. Malnutrition and low immune function not only affect the treatment effect of patients with malignant tumors, but also make malignant tumors more prone to relapse and metastasis17. More and more researches show that the nutritional status and immune function in patients with malignant tumors are closely linked to prognosis18. Compared with patients with normal nutritional status and immune function, the prognosis of those with poor nutritional status and immune function are also poor. A large number of studies have shown that immune nutrition status can be used as a powerful indicator to predict the survival outcome of patients with malignant tumors19,20,21,22.
Onodera first confirmed the prognostic role of PNI in gastrointestinal surgery of malnourished cancer patients in 198423. Recently, many studies have shown that pre-operative PNI is a good predictor of cancer prognosis after cancer surgery7,8,17,24,25,26. We in this study investigated the relatively homogeneous group of stage I–IV gastric cancer patients undergone total gastrectomy to avoid non-uniformities which undermine the scientific interpretation of PNI in reality as shown by most of the published researches.
We found that compared with patients with PNI ≥ 43, there was a significant relationship between PNI < 43 and poor median OS. After analysis and calculation, the cut-off value of pre-operative PNI was defined to be 43, which was close to the average value after the normal test. It needs to be pointed out that though there are a lot of articles about PNI published, the cut-off value of it is different in each research. This is understandable, because PNI is a dynamically changing individual indicator in the cancer microenvironment. Therefore, research of PNI in a specific cancer type, among a specific population, and under a specific state is more meaningful. Yurday et al. reported that PNI was a robust novel prognostic factor that stratifies patients with stage IIIB NSCLC and they found the cut-off value of 40.5 was statistically meaningful in the prognosis of patients survival time27. In a study about PNI prognosis in patients with high-grade serous ovarian cancer (HGSC), Zheng et al. found that a low preoperative PNI (< 45.45) was associated with an advanced FIGO stage, increased CA125 level, more extensive ascites, residual disease and platinum resistance28. In general, the cutoff value of PNI fluctuated between 45 to 57 according to the already published researches29,30,31,32,33. Refining the cutoff value for different cancer type and individual patients is an area of active research.
Although it is unclear how PNI affects the exact mechanism of cancer outcomes, the prognostic value of PNI in cancer full management is certain34,35,36. It is hypothesized that patients with high PNI may have the appropriate general conditions, as result, they can be easily presumed to have better compliance at treatment, which could make difference in long term oncologic outcomes. Gastric cancer is a malignant tumor related to digestion and nutrition. It is more significant to discuss the role of nutritional factors in the process and treatment of it. We here in this study showed TNM stage, patients age, lymph node metastasis, tumor differentiation and a low PNI were significant risk factors for a poor prognosis by univariate analyses and TNM stage, patients age and a low PNI were found to be independently associated with poor survival in multivariate analysis. When we divide the patients into non-elderly and elderly groups, significant associations were found between the PNI and factors such as lymphocyte invasion, cancer differentiation, TNM stage and tumor infiltration in non-elderly patients and the lower PNI was correlated with shorter DFS in non-elderly patients; while in elderly patients, lymphocyte invasion, cancer differentiation and TNM stage were also statistically significant and the lower PNI was correlated with shorter DFS and OS. From another point of view, nutritional parameters have been reported to be related to sensitivity to treatment37, so, the above results can help us to choose the potential beneficiary from the adjuvant treatment after total gastrectomy which is significant in clinical management.
This study included some patients with stage IV gastric cancer, and there has been no widely accepted operational indication of total gastrectomy for such patients. In a sense, the clinical characteristics and prognosis of stage IV patients are crucial to the results of this study. In general, the following patients are suitable for total gastrectomy according to our research: Firstly, the patients only have positive peritoneal cytology who are relatively mild in stage IV group, and their prognosis is significantly better than those with extensive metastases; Secondly, the patients only have proximal organ metastasis who are cautiously discussed through multidisciplinary consultation; Thirdly, the patients with metastases under special circumstances after weighing the pros and cons, for example, some patients with partial local infiltration into the pancreas body may have indications for surgery, but patients with infiltration into the pancreatic head generally lose the opportunity for surgery.
In addition to PNI, the other two commonly used nutritional parameters in the prognosis of gastric cancer are the nutritional risk index and geriatric nutritional risk index (GNRI). Both of them can be used to judge whether the patient has a good general physical condition or not. It is generally believed that patients with good nutritional status have better tolerance to the treatment and thus have a better clinical prognosis. Nutritional factors play an important role in gastric cancer prognosis and nutritional status are more likely to be a reflection of the imbalance of the tumor microenvironment in vivo.
The pre-operative PNI also has prognostic value for other types of gastrectomy. Jee et al.38 retrospectively reviewed a prospectively maintained database of 7781 gastric cancer patients who underwent gastrectomy from January 2001 to December 2010 at a single center. From that data, they analyzed clinical and pathological characteristics, PNI, and short- and long-term surgical outcomes for each patient. They found that low PNI was a poor prognostic factor for overall survival with any kind of gastrectomy and PNI can be used to predict patients at increased risk of post-operative morbidity and mortality.
Some limitations of the present study need to be noticed, Firstly, this is a retrospective study and the limited single study institute and population need to be enlarged in the future study; Secondly, patients with neo-adjuvant therapy were excluded in this study to ensure that all patients are in the same state before blood sampling, this excludes the difference between the West where neo-adjuvant treatment is routine practice and the East where neo-adjuvant treatment is not so common39. Therefore, the results of this study are not applicable to gastric patients undergoing neo-adjuvant therapy; Finally, the last one but certainly not the least one, PNI is a dynamic indicator, the previous controversy on its critical point is largely due to the difference in the nutritional status of patients in different disease states and treatment states, so it is necessary to distinguish between the early, advanced and inoperable state or different stratifications such as part resection, total resection and resection together with adjacent organs removal among the gastric cancer patients which may be more clinically valuable.
The pre-operative PNI can better reflect the surgical risk and nutritional status of gastric cancer patients. Low PNI is an independent risk factor for poor prognosis in gastric cancer patients (Supplementary Fig. 2). Therefore, patients with low pre-operative PNI levels should be observed more closely after surgery to avoid the occurrence of post-operative complications in the near future. At the same time, more detailed and closed long-term follow-up should be placed on these patients in order to obtain the opportunity to intervene in the relapse or metastasis as early as possible.
Methods
Patients
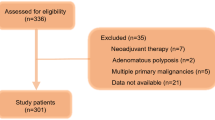
A retrospective analysis was conducted of 245 gastric cancer patients underwent total gastrectomy with R0 resection in Peking University, First Hospital between January 1st 2005 and December 30th 2015. R0 resection is defined as complete resection with negative margin. The inclusion criteria were included: (1) gastric cancer confirmed by histology and pathology; (2) clinical stage confirmed according to the 8th edition; (3) ECOG (Eastern Cooperative Oncology Group) performance status score of 0–1; (4) proportion of body/mass ≥ 20.0 kg/m2; (5) without history of other cancer; (6) no neo-adjuvant radiotherapy or chemotherapy; (7) available blood tests results collected before surgery. The exclusion criteria were included: (1) receiving any kinds of therapies before the operation; (2) pre-operative death; (3) loss of follow-up; (4) no pre-operative blood cell counts records; (5) concurrent infection; (6) autoimmune disease.
We collected the clinicopathological data and laboratory records from the patient’s case history. The patients were followed up in Peking University, First Hospital and end points for the investigation were disease-free survival (DFS) and overall survival (OS). OS was defined as the length of time from randomization to death for any reasons after total gastrectomy. DFS was defined as the time between the beginning of randomization to the recurrence of the disease or death for any causes. The end point follow-up was placed on March 2020.
Patients gave their written informed consent. The study protocol was approved by the institutional committee on human research of the Institutional Review Board (IRB) of Peking University, First Hospital. We confirm that all methods were performed in accordance with the relevant guidelines and regulations proved by IRB of Peking University, First Hospital.
Prognostic Nutritional Index (PNI)
PNI = (10 × serum Albumin, g/dL) + (0.005 × blood lymphocyte count, unit/L). Blood samples were obtained at a maximum period of 2 weeks before gastrectomy due to the half lives of albumin (≈ 21 days) and lymphocytes (> 2 weeks). The cut-off value of PNI was measured by the maximum Youden index (sensitivity + specificity − 1) in the time dependent receiver operating characteristics (ROC) curve for recurrence and survival according to published literature14.
Statistical analyses
Statistical analysis was performed using SPSS 20.0 software, chi-square test was used for comparison of probability calculation. The spearman test was used for correlation analysis. Survival rate was calculated by Kaplan–Meier survival curve, log-rank test was used for univariate analysis, and COX regression was used for multivariate analysis. p < 0.05 meant the difference was statistically significant.
References
Sugawara, K., Aikou, S., Yajima, S., Uemura, Y. & Okumura, Y. Pre- and post-operative low prognostic nutritional index influences survival in older patients with gastric carcinoma. J. Geriatr. Oncol. 11, 989–996 (2020).
Yoo, Y. J., Kang, C. M., Choi, M., Rho, S. Y. & Hwang, H. K. Pre-operative prognostic nutritional index as an independent prognostic factor for resected ampulla of Vater cancer. PLoS ONE 15, e0229597 (2020).
Wu, X., Jiang, Y., Ge, H., Diao, P. & Wang, D. Predictive value of prognostic nutritional index in patients with oral squamous cell carcinoma. Oral Dis. 26, 903–911 (2020).
Karsiyakali, N., Karabay, E. & Yucetas, U. Predictive value of prognostic nutritional index on tumor stage in patients with primary bladder cancer. Arch. Esp. Urol. 73(2), 132–139 (2020).
Migita, K., Fujii, Y., Kaji, S. & Hyakudomi, R. The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann. Surg. Oncol. 20(8), 2647–2549 (2013).
Caputo, F., Dadduzio, V., Tovoli, F., Bertolini, G. & Cabibbo, G. The role of PNI to predict survival in advanced hepatocellular carcinoma treated with Sorafenib. PLoS ONE 15(5), 223–237 (2020).
Mori, S., Usami, N., Fukumoto, K., Mizuno, T. & Kuroda, H. The significance of the prognostic nutritional index in patients with completely resected non-small cell lung cancer. PLoS ONE 10(9), 213–231 (2015).
Kanda, M., Fujii, T., Kodera, Y., Nagai, S. & Takeda, S. Nutritional predictors of post-operative outcome in pancreatic cancer. Br. J. Surg. 98(2), 268–274 (2011).
Geng, Y., Qi, Q., Sun, M., Chen, H. & Wang, P. Prognostic nutritional index predicts survival and correlates with systemic inflammatory response in advanced pancreatic cancer. Eur. J. Surg. Oncol. 41(11), 1508–1514 (2015).
Mohri, Y. et al. Prognostic nutritional index predicts post-operative outcome in colorectal cancer. World J. Surg. 37(11), 2688–2692 (2013).
Dai, Y., Fu, X., Li, T., Yao, Q. & Su, L. Long-term impact of prognostic nutritional index in cervical esophageal squamous cell carcinoma patients undergoing definitive radiotherapy. Ann. Transl. Med. 7(8), 175–187 (2019).
A-Lai, G. H., Deng, H. Y., Song, T. N., Luo, J. & Zhuo, Z. G. Pre-operative prognostic nutritional index shows no significant prognostic value for short-term outcomes of anastomosis-leakage patients after cancerous esophagectomy. Ann. Palliat. Med. 5, 698–707 (2019).
Zhang, H., Shang, X., Ren, P., Gong, L. & Ahmed, A. The predictive value of a pre-operative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J. Cell Physiol. 234(2), 1794–1802 (2019).
Komura, N., Mabuchi, S., Yokoi, E., Shimura, K. & Kawano, M. Prognostic significance of the pretreatment prognostic nutritional index in patients with epithelial ovarian cancer. Oncotarget. 38, 3605–3613 (2019).
Hofbauer, S. L. et al. The pre-operative prognostic nutritional index is an independent predictor of survival in patients with renal cell carcinoma. Urol. Oncol. 33, 68 (2015).
Galizia, G. et al. Inflammatory and nutritional status is a predictor of long-term outcome in patients undergoing surgery for gastric cancer. Validation of the Naples prognostic score. Ann. Ital. Chir. 90, 404–416 (2019).
Kosuga, T., Konishi, T., Kubota, T., Shoda, K. & Konishi, H. Value of prognostic nutritional index as a predictor of lymph node metastasis in gastric cancer. Anticancer Res. 39(12), 6843–6849 (2019).
Bai, X. & Feng, L. Correlation between prognostic nutritional index, glasgow prognostic score, systemic inflammatory response, and TNM staging in colorectal cancer patients. Nutr. Cancer. 21(2), 111–118 (2019).
Wang, Y., Zhu, Z., Li, C., Ma, Y. & You, Q. Prognostic significance of pre-operative albumin-to-globulin ratio and prognostic nutritional index combined score in Siewert type 3 adenocarcinoma of esophagogastric junction. Cancer Manag. Res. 14(11), 7631–7638 (2019).
Huang, P. Y., Wang, C. C., Lin, C. C., Lu, S. N. & Wang, J. H. Predictive effects of inflammatory scores in patients with BCLC 0-A hepatocel. J. Clin. Med. 14, 10–21 (2019).
Imai, T. et al. Pre-operative prognostic nutritional index as a method to predict post-operative complications after major head and neck surgery with free tissue transfer reconstruction. J. Clin. Oncol. 50(1), 29–35 (2020).
Shoji, F., Takeoka, H., Kozuma, Y., Toyokawa, G. & Yamazaki, K. Pretreatment prognostic nutritional index as a novel biomarker in non-small cell lung cancerpatients treated with immune checkpoint inhibitors. Lung Cancer. 136, 45–51 (2019).
Onodera, T., Goseki, N. & Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. J. Clin. Oncol. 85(9), 1001–1005 (1984).
Lin, J., Fang, T., Zhu, M., Xu, X. & Zhang, J. Comparative performance of inflammation-based prognostic scores in patients operated for intrahepatic cholangiocarcinoma. Cancer Manag. Res. 30(11), 9107–9119 (2019).
Abe, A., Kurita, K., Hayashi, H. & Ishihama, T. Correlation between prognostic nutritional index and occlusal status in gastric cancer. Oral Dis. 26(2), 465–472 (2020).
A-Lai, G. H. et al. Pre-operative prognostic nutritional index shows no significant prognostic value for short-term outcomes of anastomosis-leakage patients after cancerous esophagectomy. Ann. Palliat. Med. 8(5), 698–707 (2019).
Yurday, O. et al. Low prognostic nutritional index predicts poor clinical outcomes in patients with stage IIIB non-small-cell lung Carci. Cancer Manag. Res. 12, 1959–1967 (2020).
Zheng, F., Hao, W. & Xingzhu, J. The pre-operative prognostic nutritional index is a predictive and prognostic factor of high-grade serous ovarian cancer. BMC Cancer. 18, 883–891 (2018).
Nozoe, T., Kimura, Y. & Ishida, M. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur. J. Surg. Oncol. 28, 396–400 (2020).
Miyazaki, T., Sakai, M. & Sohda, M. Prognostic significance of inflammatory and nutritional parameters in patients with esophageal cancer. Anticancer Res. 36, 6557–6562 (2016).
Zhang, H., Shang, X. & Ren, P. The predictive value of a pre-operative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J. Cell Physiol. 234, 1794–1802 (2019).
Nakatani, M., Migita, K. & Matsumoto, S. Prognostic significance of the prognostic nutritional index in patients with recurrent esophageal squamous cell carcinoma. Nutr. Cancer. 70, 467–473 (2018).
Iguchi, T., Sugimachi, K., Mano, Y., Kono, M. & Kagawa, M. The pre-operative prognostic nutritional index predicts the development of deep venous thrombosis after pancreatic surgery. Anticancer Res. 40(4), 2297–2301 (2020).
Cheung, K., Lee, S. S. & Raman, M. Prevalence and mechanisms of malnutrition in patients with advanced liver disease, and nutrition management strategies. Clin. Gastroenterol. Hepatol. 10(2), 117–25 (2012).
Mohri, T. et al. Impact of prognostic nutritional index on long-term outcomes in patients with breast cancer. World J. Surg. Oncol. 14(1), 170–178 (2016).
Feng, J.-F. & Chen, Q.-X. Significance of the prognostic nutritional index in patients with esophageal squamous cell carcinoma. Ther. Clin. Risk Manag. 10, 1–7 (2014).
Fontanella, C., Lederer, B. & Gade, S. Impact of body mass index on neoadjuvant treatment outcome: A pooled analysis of eight prospective neoadjuvant breast cancer trials. Breast Cancer Res. Treat. 150, 127–139 (2015).
Lee, J. Y., Kim, H. I. & Kim, Y. N. Clinical significance of the prognostic nutritional index for predicting short- and long-term surgical outcomes after gastrectomy: A retrospective analysis of 7781 gastric cancer patients. Medicine (Baltimore). 95, 223–240 (2016).
Aydın, Y. et al. Prognostic importance of serum CRP, prealbumin, and transferrin levels in patients with advanced stage esophageal cancer. JTJTCS. 19(3), 384–390 (2011).
Acknowledgements
We thanks a lot for the contribution of Dr. Pan Jianhong for his statistical help for this article.
Funding
The financial support for study comes from the Youth Science Grant of Peking University, First Hospital.
Author information
Authors and Affiliations
Contributions
Z.X. design the research and write the article; Z.Y. and M.F. collect the data; X.L. do work on the data analysis and W.S. review and correct the whole article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xishan, Z., Ye, Z., Feiyan, M. et al. The role of prognostic nutritional index for clinical outcomes of gastric cancer after total gastrectomy. Sci Rep 10, 17373 (2020). https://doi.org/10.1038/s41598-020-74525-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-74525-8
This article is cited by
-
Preoperative prognostic nutritional index value as a predictive factor for postoperative delirium in older adult patients with hip fractures: a secondary analysis
BMC Geriatrics (2024)
-
Effect of Preoperative Body Mass Index on Postoperative and Long-Term Outcomes in an East Indian Gastric Cancer Cohort
Journal of Gastrointestinal Cancer (2024)
-
Association of prognostic nutritional index with long-term mortality in patients receiving percutaneous coronary intervention for acute coronary syndrome: a meta-analysis
Scientific Reports (2023)
-
Differential prognostic significance of sarcopenia in metastatic esophageal squamous and adenocarcinoma
Esophagus (2023)
-
Is the Prognostic Nutritional Index a Prognostic Marker for the Survival of Patients with Lymph-Node Positive Stage II-III Gastric Cancer Who Receive Adjuvant Chemotherapy?
Journal of Gastrointestinal Cancer (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.