Abstract
Acute coronary syndrome (ACS) is closely associated with an increased risk of death. Nrg4, a novel adipocytokine, has negative correlations with indicators of metabolic syndrome. Here, we investigated whether circulating Nrg4 associates with the prevalence of ACS. In this case-control study, a total of 257 subjects (144 patients with ACS and 56 patients diagnosed with stable angina pectoris (SAP)) compared to 57 healthy controls. Serum Nrg4 and hs-CRP concentrations were determined by ELISA. The associations of circulating Nrg4 with other clinical parameters were also analyzed. Serum levels of Nrg4 were lower in patients compared to the control subjects (0.7 ± 0.53 ng/mL versus 1.1 ± 0.9 ng/mL, P = 0.018). There was a significant association between higher Nrg4 level and lower risk of ACS (OR = 0.15; 95%CI = 0.02–0.9; P = 0.046), but not with SAP. This association was independent of potential confounders including traditional cardiovascular risk factors. The distribution of patients with no, 1, 2 and 3 vessel stenosis was significantly different in Nrg4 quartiles. Patients in the lower quartile of Nrg4 were more likely to experience 3 vessel diseases. Serum levels of Nrg4 correlated negatively with HDL-cholesterol in ACS patients. Decreased serum levels of Nrg4 might be an independent risk factor for ACS.
Similar content being viewed by others
Introduction
Coronary artery disease (CAD) still remains the leading cause of morbidity and mortality worldwide1. Identifying protective and risk factors may guide the new strategies for prevention of CAD. Adipocytokines are bioactive molecules, which are secreted by adipose tissue and gaining increasing attention as risk factors candidates of CAD. Previous research has indicated both pro- and anti-inflammatory adipocytokines, including leptin and adiponectin, were associated with the incidence of CAD2. Among these proteins is neuregulin 4 (Nrg4), a novel adipocytokine, which is mainly secreted by brown adipose tissue. This adipocytokine is a member of the epidermal growth factor (EGF) family of extracellular ligands, which specifically activates EGF receptor ErbB4 (v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 4) to improve systemic energy metabolism3 and preserve glucose homeostasis3,4,5.
Nrg4 has potent liver antilipogenic activity6 and its expression is reduced in mouse and human obesity4. Nrg4 concentration has negative correlations with indicators of metabolic syndrome7. Nrg4 deficiency6 and its impaired signaling exacerbated glucose intolerance, insulin resistance and diet-induced hepatic steatosis4. Recently, it has been shown that Nrg4 signaling pathway has a role in inhibition of endothelial cell apoptosis and prevention of atherosclerosis progression8,9. Jiang et al. study showed that the individuals with increased carotid intima-media thickness (CIMT) and carotid plaques had lower Nrg4 concentrations5.
Based on the aforementioned researches concerning the association between Nrg4 and multiple cardiovascular risk factors, circulating Nrg4 might be a potential candidate marker of ACS risk. However, information about the link between Nrg4 and CAD is scarce. Accordingly, in the current study, we aimed to explore the relationship between serum Nrg4 levels and the risk of acute coronary syndrome (ACS) in Iranian CAD patients. Additionally, the association between serum Nrg4 levels and various risk factors for ACS was also evaluated.
Results
Clinical characteristics of the study population
The demographic and clinical characteristics of the study subjects (n = 257) are shown in Table 1. In total, 123 males and 134 females were included. Subjects were categorized to 3 groups. The mean age of the subjects was 61.3 ± 9.1 years in SAP, 59.9 ± 12.5 years in ACS and 45.6 ± 11.1 years in control (P = 0.001). Distribution of gender and smoking was significantly different between study groups (P < 0.05). Waist, systolic blood pressure, levels of fasting glucose and triglyceride were significantly higher and HDL and cholesterol were significantly lower in SAP and ACS compared to the control subjects (P < 0.05).
Serum hs-CRP and Nrg4 level in study subjects
As shown in Table 1, serum hs-CRP level was compared between SAP, ACS and control group. Hs-CRP serum level was significantly higher in ACS patients compared to SAP patients (262 ± 198.8 μg/mL versus 185 ± 144.7 μg/mL, P = 0.047) and control subjects (262 ± 198.8 μg/mL versus 147.6 ± 137.1 μg/mL, P = 0.001). Nrg4 serum level was compared in patients and the healthy control group. As was shown in Fig. 1A, serum Nrg4 level was significantly higher in control compared to CAD patients (SAP + ACS) (1.1 ± 0.9 ng/mL versus 0.7 ± 0.53 ng/mL, P = 0.018). Comparison of Nrg4 level between SAP, ACS and healthy controls revealed that Nrg4 was significantly lower in ACS group compared to controls (0.56 ± 0.23 ng/mL versus 1.1 ± 0.9 ng/mL, P = 0.02). No significant differences were observed between control and SAP and between SAP and ACS groups (Fig. 1B).
Association of Nrg4 with other factors
Multivariate linear-regression analysis was carried out to identify independent associations of hs-CRP, gensini score and cardiovascular risk factors with Nrg4 serum level in both ACS and SAP patients (Table 2). No significant differences were observed (P ≥ 0.05). In ACS patients, serum Nrg4 levels showed a significant negative association with HDL cholesterol (β = −0.5, P = 0.04).
Relationship between serum Nrg4 concentrations and the presence of the coronary disease
The associations of serum Nrg4 levels with an increased risk of coronary syndrome were assessed. The results of multinomial regression analyses and odds ratios (ORs) for the association of serum Nrg4 levels with SAP and ACS disease are shown in Table 3. Cardiovascular risk factors were included in 3 different regression models and adjusted odds ratios along with crude ORs were reported. Comparing SAP patients with the control group, lower Nrg4 serum level was independently related to higher risk of the disease (OR = 0.45; 95%CI = 0.2–0.9; P = 0.03) when adjusted for age, gender, BMI, waist, systolic blood pressure and smoking. However, there was no significant association between serum Nrg4 level and SAP after including other variables HDL, LDL, triglyceride, total cholesterol and FBS in the regression model (P > 0.05). The results of comparison of Nrg4 levels between ACS and control subjects revealed that there was a significant association between higher Nrg4 level and lower risk of ACS. This association was observed in both crude OR analyses (OR = 0.57; 95%CI = 0.37–0.89; P = 0.01) and after adjustment for age, gender, BMI, waist, systolic blood pressure and smoking (OR = 0.3; 95%CI = 0.15–0.7; P = 0.003), HDL, LDL, triglyceride, total cholesterol, FBS (OR = 0.4; 95%CI = 0.2–0.8; P = 0.004), age, gender, BMI, waist, systolic blood pressure, smoking, HDL, LDL, triglyceride, total cholesterol and FBS (OR = 0.15; 95%CI = 0.02–0.9; P = 0.046).
Analysis of the serum Nrg4 quartiles with clinical characteristics
Table 4 shows clinical and demographic characteristic of ACS and control subjects by quartiles of serum Nrg4 levels. No significant differences were observed in age, gender, BMI, waist, LDL cholesterol, total cholesterol, triglyceride, fasting glucose, current smoker and hs-CRP among four quartiles of serum Nrg4 levels in both control and ACS patients. Control subjects with the lowest quartile of Nrg4 had significantly higher HDL cholesterol compared to the third (60.9 ± 25.6 versus 40.2 ± 9.9; P = 0.02) and fourth (60.9 ± 25.6 versus 42.2 ± 7.8; P = 0.04) quartiles. Nrg4 serum concentration was significantly different between all quartiles in ACS and control subjects (P ˂0.0001) (Table 4).
Number of vessels with coronary stenosis in all patients containing SAP and ACS were compared in Nrg4 quartiles. Analysis revealed that the distribution of patients with no, 1, 2 and 3 vessel stenosis was significantly different in Nrg4 quartiles (P = 0.017). As shown in Fig. 2, patients with the lowest quartile of Nrg4, 14.3% had no vessel disease, 17.9% had 1 vessel stenosis, 28.6% had 2 vessel diseases and 39.3% had 3 vessels stenosis. In patients with the highest quartile of Nrg4, 54.8% were detected with no vessel stenosis while16.1% had 1 vessel, 12.9% had 2 vessel and 16.1% had 3 vessel stenosis. Distribution of patients in quartile 2 and 3 of Nrg4 are also shown in Fig. 2.
Serum quartiles of Nrg4 compared according to the vessel stenosis. Nrg4 quartile 1 (≤0.09 ng/mL) (white column), Nrg4 quartile 2 (0.0901–0.31 ng/mL) (dotted column), Nrg4 quartile 3 (0.3101–1.37 ng/mL) (dashed column) and Nrg4 quartile 4 (>0.137 ng/mL) (black column). No VD = no vessel disease, 1 VD = single vessel disease, 2 VD = double vessel disease, 3 VD = triple vessel disease.
Discussion
In the present study, we found a negative association between serum Nrg4 levels and the risk of acute coronary syndrome (ACS) (including acute myocardial infarction (AMI) + unstable angina pectoris (UAP)) but not stable angina pectoris (SAP). This association was independent of potential confounders, including traditional cardiovascular risk factors. Additionally, we demonstrated a negative association between the level of Nrg4 and the number of diseased vessels (Fig. 2). To the best of our knowledge, this is the first study evaluating serum Nrg4 levels in ACS patients.
These findings are in line with Tian clinical study in 73 CAD patients, which were divided into two subgroups according to their SYNTAX score. The results showed that patients with a higher SYNTAX score had lower plasma concentration of Nrg410. Our findings are also in agreement with the Jiang et al. research regarding the role of Nrg4 in subclinical cardiovascular disease. They measured the Nrg4 levels in 485 obese adult subjects and adjusting for sex,age, systolic BP, fasting glucose, alcohol consumption, physical activity, BMI, current smoking, total cholesterol, HDL-c, body fat and HOMA-IR, they found that the individuals in the lowest quartile of serum Nrg4 had a 3.7 fold increased risk of increased CIMT and 2.06 fold increase in atherosclerotic plaques5.
Our finding about the cardio protective effects of Nrg4 may be attributed to a number of direct and indirect mechanisms. There are intriguing animal studies and clinical trials, which show a direct connection between Nrg4 associated downstream signaling pathway, the ErbB4, and cardiomyocyte proliferation11,12. This signaling pathway not only controls cardiomyocyte generation, differentiation and migration13, but also plays a critical role in repair of heart injury14,15. McBride et al. study showed that some ErbB4 gene variants are associated with congenital heart disease consisting of left-sided obstructive lesions16. Clement et al. showed that ErbB4 was upregulated following balloon injury to the rat carotid artery17.
On the other hand, there are indirect mechanisms, which can justify the protective role of Nrg4 in ACS. Increasing evidence has highlighted the Nrg4 distinct biological effects on traditional cardiovascular risk factors, including adiposity, lipoprotein metabolism, insulin resistance and inflammation4,18. Wang et al. revealed that the adipose Nrg4 mRNA expression inversely correlates with adiposity. Nrg4 inhibits lipogenesis by suppressing Srebp1c gene, a key regulator of lipogenesis and triglyceride synthesis, and induces a catabolic metabolic state in the liver. They showed that transgenic expression of Nrg4 recovers diet-induced metabolic disorders. On the other hand, Nrg4 improves insulin sensitivity by enhancing peripheral glucose metabolism and insulin resistance4.
Some studies support the beneficial role of Nrg4 in the prevention and management of inflammation7,19. Inflammation plays a key role in coronary atherosclerotic plaque destabilization and ACS development20. Activated macrophages and T-cells within plaques, which produce a range of proinflammatory mediators including interleukin (IL)-1 and IL-6, interferon (IFN)-γ, tumor necrosis factor (TNF)α are main perpetrators of proteolytic destruction of smooth muscle cells21,22,23 and connective tissue matrix24. The anti-inflammatory effect of Nrg4 was reported in experimental colitis mice. Schumacher et al. showed that the administration of exogenous Nrg4 stimulates colonic pro-inflammatory macrophage apoptosis and ameliorates inflammation14. Yan et al. study confirmed that the plasma Nrg4 levels negatively correlated with hs- CRP level, WBC count, and neutrophil count in nT2DM patients7. Ma et al. showed that Nrg4 overexpression enhanced the apoptosis of proinflammatory macrophage and reduced expression of macrophage markers including F4/80, Cd68, Cd11b and Cd11c genes and macrophage chemokine gene, Mcp118.
Another interesting finding of the study was the negative correlation between HDL-cholesterol and serum levels of Nrg4 in ACS patients. Although this finding supports the relation between nrg4 and lipoprotein metabolism, but the discrepancy between our results and previous reports7,10 and lack of physiological mechanisms underlying the relationship between Nrg4 and HDL-C prevents us from drawing conclusions about this finding.
Taken together, on the basis of our findings about the connection between Nrg4 levels, risk of ACS and the number of diseased vessels and the aforementioned findings about the role of Nrg4 in preserving structural and functional integrity of adult heart25 and metabolic homeostasis4, one can assume that this new adipocytokine exerts protective effects in ACS. However, our study has some limitations. Firstly, our investigation represents a case-control study which cannot establish causality. Secondly, our study was based on CAD patients at a single center. Further prospective and population-based studies are needed to verify the potential causal relationship between the Nrg4 levels and ACS.
In summary, the present paper, apparently for the first time, suggests that Nrg4 deficiency is a potential risk factor that directly and indirectly may contribute to the incidence of ACS and proposes that Nrg4 may be a useful biomarker for predicting CAD outcomes. Our study may serve as a basis for future studies to explore the potential preventive and therapeutic effects of Nrg4 on ACS.
Materials and methods
Study population
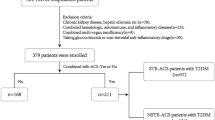
In this case-control study, 200 CAD patients who underwent diagnostic coronary angiography between March 2018 and March 2019 in Jorjani heart center, Bandar Abbas, Iran, were compared with 57 healthy controls. Patients were divided into 2 groups: Stable angina pectoris (SAP) (n = 56) patients were selected based on the Canadian Cardiovascular Society Classification System, in which class 1 or 2 were included as SAP26 and ACS groups (n = 144). ACS classification performed based on electrocardiogram (ECG) and troponin measurement for those patients presenting to the emergency room with chest discomfort. Included patients were those who were diagnosed with unstable angina pectoris (UAP) (those who classified in Canadian Cardiovascular Society class 3 or 4 with ischemic discomfort, without electrocardiographic ST-segment elevation and elevation of troponin) and AMI (with ischemic discomfort, with or without ST-segment elevation on the ECG, and elevation of troponin level). All of them were enrolled in ACS group. The healthy control group consisted of 57 subjects with no history of cardiovascular disease. Exclusion criteria were as follows: Patients who received immune system affecting drugs, those with history of thyroid dysfunction, autoimmune diseases, infectious diseases and severe disorders of the liver, kidney and brain and patients with valvular heart disease and malignant disease. All patients signed written informed consent. The study was approved by the ethical committee of the Hormozgan University of Medical Sciences (the committee’s reference number: 960248). Cardiovascular risk factors including blood pressure, and smoking were recorded. Body mass index was computed as weight (Kg) divided by height squared (m2). Smoking was defined as the use of >1 cigarettes/day at the time of diagnosis. Waist circumference was measured at the level of the umbilicus. Measurements were repeated three times and the mean values were used for analysis.
Blood sampling and biochemical measurement
Venous blood was drawn from all subjects after admission in a fasting state. After coagulation at room temperature for 30 min, centrifugation was performed at 2000 g for 15 min at 4 °C (sigma 2–16 KL) and serum samples were stored at −80 °C for the subsequent analysis. Total cholesterol, triglyceride, low and high-density lipoprotein, cholesterol and blood glucose were analyzed in a biochemical autoanalyzer (Hitachi 7600, Japan).
Serum Nrg4 and hs-CRP measurement
Serum Nrg4 was measured using enzyme-linked immunosorbent assay (ELISA) kit (Zell bio, Germany). The sensitivity of the kit was (0.02 ng/mL). Standard curve was developed by a linear range of the standard and used for the calculation of Nrg4 concentration. The intra and inter assay variations were <10%. ELISA kit was used for serum level of hs-CRP measurement (Zell bio, Germany, [CV], <10%). The linear range of the standard was 0.1–10 µg/mL. The lowest detectable limit of hs-CRP was 0.01 µg/mL.
Coronary angiography
Coronary angiography was performed by professionals with a femoral approach using the Judkins technique and two blinded cardiologists judged the results. Coronary stenosis was accepted if more than 50% stenosis was seen in at least one coronary artery. The severe part of the lesion was used for calculation of stenosis diameter. Patients with new-onset acute chest pain were divided to single-vessel, double-vessel, and triple-vessel diseases based on their coronary angiographic results and the severity of coronary atherosclerosis and the patients with normal coronary arteries or vessel stenosis less than 50% were considered as no-vessel disease group.
Gensini score
After performing coronary angiography, the severity of coronary stenosis was calculated using Gensini score estimation as defined previously27.
Statistical analysis
All statistical analyses were performed with the Statistical Package for Social Sciences version 16.0 (SPSS Inc., Chicago, IL, USA). Demographic and clinical data was compared in SAP, ACS and control groups using one-way ANOVA and presented as mean ± SD. Categorical variables were displayed numerical and percentile and compared using chi-square test. Analyses of serum Nrg4 levels and metabolic risk factors performed using general linear regression analyses and standardized regression coefficient was reported. Multivariable logistic regression models were used to examine the association of serum Nrg4 levels with the disease. Odds ratio and 95% confidence interval were reported and adjusted for other covariates. Results were considered statistically significant at two-sided P < 0.05.
Ethical approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. The ethical committee of the Hormozgan University of Medical Sciences confirmed the study (the committee’s reference number: 960248).
References
Okrainec, K., Banerjee, D. K. & Eisenberg, M. J. Coronary artery disease in the developing world. American heart journal 148(1), 7–15 (2004).
Cai, C. et al. Association of circulating neuregulin 4 with metabolic syndrome in obese adults: a cross-sectional study. BMC medicine 14(1), 165 (2016).
Yan, P. et al. Decreased plasma neuregulin 4 levels are associated with peripheral neuropathy in Chinese patients with newly diagnosed type 2 diabetes: A cross-sectional study. Cytokine 113, 356–364 (2019).
Wang, G.-X. et al. The brown fat–enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nature medicine 20(12), 1436 (2014).
Jiang, J. et al. Circulating neuregulin 4 levels are inversely associated with subclinical cardiovascular disease in obese adults. Scientific reports 6, 36710 (2016).
Feve, B. et al. New adipokines. Ann Endocrinol (Paris) 77(1), 49–56 (2016).
Yan, P. et al. Plasma neuregulin 4 levels are associated with metabolic syndrome in patients newly diagnosed with type 2 diabetes mellitus. Disease markers, 2018. 2018.
Qin, M. et al. Myricitrin attenuates endothelial cell apoptosis to prevent atherosclerosis: An insight into PI3K/Akt activation and STAT3 signaling pathways. Vascular pharmacology 70, 23–34 (2015).
Tie, G. et al. Oxidized low-density lipoprotein induces apoptosis in endothelial progenitor cells by inactivating the phosphoinositide 3-kinase/Akt pathway. J. Vasc Res 47(6), 519–30 (2010).
Tian, Q.-P. et al. Association of circulating neuregulin-4 with presence and severity of coronary artery disease. International heart journal, 18–130 (2019).
Bersell, K. et al. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 138(2), 257–270 (2009).
Iwamoto, R. et al. Heparin-binding EGF-like growth factor and ErbB signaling is essential for heart function. Proceedings of the National Academy of Sciences 100(6), 3221–3226 (2003).
Wadugu, B. & Kühn, B. The role of neuregulin/ErbB2/ErbB4 signaling in the heart with special focus on effects on cardiomyocyte proliferation. American Journal of Physiology-Heart and Circulatory Physiology 302(11), H2139–H2147 (2012).
Schumacher, M. A. et al. ErbB4 signaling stimulates pro-inflammatory macrophage apoptosis and limits colonic inflammation. Cell death & disease 8(2), e2622 (2017).
Galindo, C. L., Ryzhov, S. & Sawyer, D. B. Neuregulin as a heart failure therapy and mediator of reverse remodeling. Current heart failure reports 11(1), 40–49 (2014).
McBride, K. L. et al. Association of common variants in ERBB4 with congenital left ventricular outflow tract obstruction defects. Birth Defects Research Part A: Clinical and Molecular Teratology 91(3), 162–168 (2011).
Clement, C. M. et al. Neuregulin-1 attenuates neointimal formation following vascular injury and inhibits the proliferation of vascular smooth muscle cells. Journal of vascular research 44(4), 303–312 (2007).
Ma, Y., Gao, M. & Liu, D. Preventing high fat diet-induced obesity and improving insulin sensitivity through neuregulin 4 gene transfer. Scientific reports 6, 26242 (2016).
Chen, Z. et al. Nrg4 promotes fuel oxidation and a healthy adipokine profile to ameliorate diet-induced metabolic disorders. Molecular metabolism 6(8), 863–872 (2017).
Pasqui, A. et al. Pro-inflammatory/anti-inflammatory cytokine imbalance in acute coronary syndromes. Clinical and experimental medicine 6(1), 38 (2006).
Kishikawa, H., Shimokama, T. & Watanabe, T. Localization of T lymphocytes and macrophages expressing IL-1, IL-2 receptor, IL-6 and TNF in human aortic intima. Role of cell-mediated immunity in human atherogenesis. Virchows Archiv A 423(6), 433–442 (1993).
Kato, K. et al. Elevated levels of pro-inflammatory cytokines in coronary artery thrombi. International journal of cardiology 70(3), 267–273 (1999).
Zhou, X. CD4+ T cells in atherosclerosis. Biomed Pharmacother 57(7), 287–91 (2003).
Moreno, P. R. et al. Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture. Circulation 90(2), 775–778 (1994).
Xu, Y. et al. Neuregulin-1/ErbB signaling and chronic heart failure, in Advances in Pharmacology, Elsevier. p. 31–51 (2010).
Gibbons, R. J. et al. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Journal of the American College of Cardiology 40(8), 1531–1540 (2002).
Gensini, G. G. A more meaningful scoring system for determining the severity of coronary heart disease. Am J cardiol 51, 606 (1983).
Acknowledgements
We extend our thanks to Ms. Gisoo Mehri for her useful comments on our manuscript and the research council of Hormozgan University of Medical Sciences for their financial support. The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
N.N. and M.R. developed the concept and prepared the manuscript; N.F. performed experimentation, H.M. and H.F. performed examination on patients and operation, M.R. analyzed data, prepared tables and carefully read the manuscript. All authors read and approved the final article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rahimzadeh, M., Farshidi, N., Naderi, N. et al. Clinical significance of serum concentrations of neuregulin-4, in acute coronary syndrome. Sci Rep 10, 5797 (2020). https://doi.org/10.1038/s41598-020-62680-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-62680-x
This article is cited by
-
Circulating expression patterns of TL1A and FFAR2 in patients with stable and unstable angina
Egyptian Journal of Medical Human Genetics (2023)
-
Beiging of perivascular adipose tissue regulates its inflammation and vascular remodeling
Nature Communications (2022)
-
Neuregulin 4 Attenuates Osteoarthritis Progression by Inhibiting Inflammation and Apoptosis of Chondrocytes in Mice
Calcified Tissue International (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.