Abstract
The response of plant root development to nutrient deficiencies is critical for crop production. Auxin, nitric oxide (NO), and strigolactones (SLs) are important regulators of root growth under low-nitrogen and -phosphate (LN and LP) conditions. Polar auxin transport in plants, which is mainly dependent on auxin efflux protein PINs, creates local auxin maxima to form the basis for root initiation and elongation; however, the PIN genes that play an important role in LN- and LP-modulated root growth remain unclear. qRT-PCR analysis of OsPIN family genes showed that the expression of OsPIN1b is most abundant in root tip and is significantly downregulated by LN, LP, sodium nitroprusside (SNP, NO donor), and GR24 (analogue of SLs) treatments. Seminal roots in ospin1b mutants were shorter than those of the wild type; and the seminal root, [3H]IAA transport, and IAA concentration responses to LN, LP, SNP, and GR24 application were attenuated in ospin1b-1 mutants. pCYCB1;1::GUS expression was upregulated by LN, LP, SNP, and GR24 treatments in wild type, but not in the ospin1b-1 mutant, suggesting that OsPIN1b is involved in auxin transport and acts as a downstream mediator of NO and SLs to induce meristem activity in root tip in rice under LN and LP.
Similar content being viewed by others
Introduction
Nitrogen (N) and phosphate (P) are required for plant growth and development; however, the natural supply of soil N and P limits plant growth and development in most agricultural cropping systems1,2. Therefore, the response of the root system architecture to N and P deficiencies is critical for plant growth and crop productivity. Increases in the root-to-shoot ratio and root surface area regulated by deficiencies of N and P have been studied in several plant species3,4,5, most of which focused on the responses of the root system to P deficiency. Two contrasting reactions of root growth to P deficiency have been recorded: 1) reduction of primary root growth and enhancement of lateral root (LR) density in Arabidopsis3,6,7,8,9; and 2) elongation of primary roots in other plant species (such as rice)10,11, suggesting that P deficiency-related changes in the root morphology are complex and vary according to the experimental conditions and plant species. Moreover, in maize and rice, elongation of primary roots and inhibition of LR are typical responses to N deprivation4,12,13,14.
Root architecture is a highly plastic trait, and its plasticity is strongly controlled by environmental conditions and plant regulators. Polar auxin transport and auxin signaling pathways play essential roles in root development15,16,17. Polar auxin transport in plants creates local auxin maxima that form the basis for root initiation and elongation18,19,20. Polar auxin transport is mainly mediated by auxin efflux carrier PIN family proteins21. Some lines of evidence suggest that the OsPIN family genes participate in root formation and elongation in rice22,23,24,25. Moreover, the inhibited auxin transport from the shoot to root, which is largely dependent on reduced expression of OsPIN genes, plays an important role in low nitrogen (LN)- and low phosphate (LP)-modulated LR formation and seminal root elongation in rice12. Few studies have assessed the PIN genes involved in auxin polar transport and LN- and LP-mediated modulation of root development.
Root development involves cross-talk among several plant hormones. Auxins play an important role in regulating root growth and development under low N and low P conditions12. In addition to auxin, strigolactones (SLs) and nitric oxide (NO) are associated with the regulation of root development12,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42. Several lines of evidence suggest that the SL pathway is involved in rice root elongation under LN and LP conditions12,34. NO, as a signaling molecule, plays a pivotal role in root growth modulation43. NO participates in LN- and LP-induced seminal root elongation in rice43. SL signaling genes are required for NO-induced elongation of seminal root in response to LN and LP in rice plants43. Moreover, SLs are believed to modulate auxin transport to regulate root formation and elongation12,32,33. Although NO is closely associated with auxin by regulating PIN family genes and root elongation in plants30, whether auxin transport plays a role in NO-modulated root elongation under LN- and LP-deficient conditions remains unclear.
In this study, phenotypic, cellular, and genetic analyses of rice were performed to explore the role of the OsPIN gene in regulating auxin polar transport and root development, and the crosstalk between SLs and NO under LN and LP conditions. We found that SLs are required for NO-mediated modulation of LN- and LP-induced seminal root elongation (similar responses of seminal and adventitious roots to LN and LP) and function by inhibiting auxin transport to the root tip. Of 10 OsPINs, the expression of OsPIN1b, which encodes an auxin efflux carrier, was highest in rice root tip, and so might be involved in auxin transport and elongation of seminal root by regulating meristem activity in the root tips of rice plants under LN and LP conditions.
Results
NO regulates root elongation by inhibiting polar auxin transport
LN- and LP-enhanced NO production resulted in the elongation of seminal roots (Supplementary Fig. S1a,c,d), as reported previously44. The effects of NO on auxin status in rice were investigated using transgenic plants expressing the pDR5::GUS construct. Compared with the control, pDR5::GUS intensity and IAA concentrations decreased in LN- and LP-treated root tips of WT plants (Supplementary Fig. S1b–e). The application of sodium nitroprusside (SNP, NO donor) under normal nutrition conditions decreased pDR5::GUS expression and IAA concentration in root tips to a level similar as that under nutrient-deficient conditions. Application of cPTIO (2-(4-carboxyphenyl)-4,4,5,5- tetramethylimidazoline-1-oxyl-3-oxide, NO scavenger) under low-nutrient conditions markedly increased pDR5::GUS expression and IAA concentration in root tips to a level similar to that under control conditions (Supplementary Fig. S1b–e). However, pDR5::GUS expression and seminal root length showed no differences between control and control + cPTIIO (Fig. S1f–h), suggesting that the LN- and LP-mediated increase in NO levels reduced auxin levels in rice root tips.
As shown in Fig. 1a,b, the application of naphthylacetic acid (NAA; an analogue of IAA) under LN and LP conditions reduced seminal root length and increased pDR5::GUS expression to levels similar to those under normal nutrition conditions. Moreover, application of NAA and SNP under control conditions markedly relieved the inductive effect of SNP application on seminal root elongation and its inhibitory effect on pDR5::GUS expression. Therefore, NO modulates LN- and LP-induced seminal root elongation by reducing auxin levels. In rice plants treated with N-1-naphthylphthalamic acid (NPA, a polar auxin transport inhibitor) under control conditions, the length of seminal roots markedly increased and the pDR5::GUS expression in the root tip significantly decreased to levels similar to those under low-nutrient conditions. Furthermore, application of NPA and cPTIO under LN and LP conditions suppressed the inhibitory effect of cPTIO [2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, an scavenger of NO] on seminal root elongation and its inductive effect on pDR5::GUS expression. These results suggested that NO negatively regulates auxin polar transport and reduces auxin levels in the root tip.
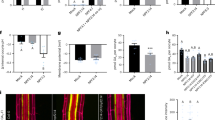
The seminal root length and histochemical localization of pDR5::GUS expression in the root tip of wild-type (WT) rice seedlings (Shiokari). Seedlings were grown in hydroponic medium containing normal nutrition (control; 2.5 mM N, 300 μM P), low N, or low P (LN, 0.02 mM; LP, 2 µM), or subjected to treatment with sodium nitroprusside (SNP, 10 µM), 2-(4-carboxyphenyl)-4,4,5,5- tetramethylimidazoline-1-oxyl-3-oxide (cPTIO, 80 µM), or a-naphthylacetic acid (NAA, 10 nM), or localized application of N-1-naphthylphthalamic acid (NPA, 20 µM) by dispensing diluted agar onto the root-shoot junction for 14 days. (a) Seminal root length; (b) histochemical localization of pDR5::GUS expression in root tip. Bar = 1 mm. Data are means ± SE of eight replicates and bars with different letters indicate significant differences at p < 0.05, as determined by ANOVA followed by the LSD test.
We evaluated the effects of SNP, cPTIO, and GR24 (an analogue of SLs) on [3H]IAA transport (Fig. 2a). [3H]IAA activity was lower in LN- and LP-treated root tips than in the control, suggesting that LN and LP decreased [3H]IAA transport from shoots to roots. Moreover, application of SNP and GR24 under control conditions markedly reduced [3H]IAA transport to root tips to the same extent as LP and LN; conversely, treatment with cPTIO under LN and LP conditions markedly increased [3H]IAA transport to root tips to the same extent as under control conditions (Fig. 2a). Quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis showed that OsPIN1a-b, OsPIN2, and OsPIN10a-b expression levels in WT root were significantly reduced by SNP treatment compared with those in the control (Fig. 2b). OsPIN1b expression was downregulated by LN and LP and restored by cPTIO in WT roots (Fig. 2c). These findings confirmed that NO modulates LN- and LP-induced seminal root elongation by suppressing polar auxin transport.
[3H]IAA transport and PIN family expression in root of WT rice seedlings (Shiokari). Seedlings were grown in hydroponic medium containing normal nutrition (control; 2.5 mM N, 300 μM P), low N, or low P (LN, 0.02 mM; LP, 2 µM P), or subjected to treatment with sodium nitroprusside (SNP, 10 µM), 2-(4-carboxyphenyl)-4,4,5,5- tetramethylimidazoline-1-oxyl-3-oxide (cPTIO, 80 µM), or GR24 (analog of SLs, 2.5 µM) for 14 days. (a) [3H]IAA transport; (b) PIN family expression; and (c), OsPIN1b expression. Data are means ± SE of eight replicates (a) and three replicates (b,c). Bars with different letters indicate significant differences at p < 0.05, as determined by ANOVA followed by the LSD test.
Strigolactones and strigolactone signaling are required for NO-mediated suppression of polar auxin transport
SLs are required for the role of NO in LN- and LP-induced seminal root elongation43. Under control conditions, application of SNP or GR24 reduced pDR5::GUS expression in WT root tips (Fig. 3). However, LN and LP treatments and application of SNP did not affect pDR5::GUS expression in SL synthetic and signaling mutants (d10 and d3). Compared with control conditions, no change in [3H]IAA transport from shoots to root tips was observed in the d10 mutant under low-nutrient conditions with the application of cPTIO (Fig. 4a). However, application of GR24 under control conditions markedly reduced [3H]IAA transport to root tips (Fig. 4a). No difference in the expression of OsPIN family genes was observed in the roots of the d10 mutant between control and SNP conditions (Fig. 4b, Supplementary Fig. S2). In comparison to control conditions, OsPIN1b expression in the d10 mutant was unchanged under LN, LP, and cPTIO treatments (Fig. 4c). These results confirmed that SLs and SL signaling are required for NO-mediated suppression of polar auxin transport in rice plants under LN and LP conditions.
Histochemical localization of pDR5::GUS expression in root tip. Seedlings were grown in hydroponic medium containing normal nutrition (control; 2.5 mM N, 300 μM P), low N, or low P (LN, 0.02 mM; LP, 2 µM), or subjected to treatment with sodium nitroprusside (SNP, 10 µM), 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO, 80 µM), or GR24 (analog of SLs, 2.5 µM) for 14 days. Bar = 1 mm; n = 8.
[3H]IAA transport and PIN family expression in root of the d10 mutant. Seedlings were grown in hydroponic medium containing normal nutrition (control; 2.5 mM N, 300 μM P), low N, or low P (LN, 0.02 mM; LP, 2 µM) in addition to sodium nitroprusside (SNP, 10 µM), 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO, 80 µM), or GR24 (analog of SLs, 2.5 µM) for 14 days. (a) [3H]IAA transport; (b) PIN family expression; and (c) OsPIN1b expression. Data are means ± SE of eight replicates (a) and three replicates (b,c). Data are means ± SE of eight replicates and bars with different letters indicate significant differences at p < 0.05, as determined by ANOVA followed by the LSD test.
OsPIN1b is involved in NO- or SL-induced root elongation under LN and LP conditions
We analysed 10 OsPIN family genes in root tip of rice plants (Supplementary Fig. S3). Relative expression of OsPIN1b was the highest of the OsPINs in rice root tip, consistent with a previous study of other rice cultivars44. Therefore, OsPIN1b was used as the target gene in subsequent analyses. Compared with control conditions, OsPIN1b expression in root tip was significantly reduced by SNP treatment; however, application of abamine (an SL inhibitor) ameliorated this effect (Fig. 5a). Moreover, OsPIN1b expression was significantly upregulated by cPTIO application under LN or LP conditions in the WT compared with mock treatments, to a level similar to that in the d10 mutant under LN and LP conditions (Fig. 5b,c).
OsPIN1b expression and localization of pPIN1b::GUS expression in root tip. Seedlings were grown in hydroponic medium containing normal nutrition (control; 2.5 mM N, 300 μM P), low N, or low P (LN, 0.02 mM; LP, 2 µM ), or subjected to treatment with sodium nitroprusside (SNP, 10 µM), 2-(4-carboxyphenyl)-4,4,5,5- tetramethylimidazoline-1-oxyl-3-oxide (cPTIO, 80 µM), GR24 (analog of SLs, 2.5 µM), or abamine (100 µM) for 14 days. (a–c) OsPIN1b expression under control (a), LN (b), and LP (c) conditions. (d) Localization of pPIN1b::GUS expression. mRNA levels were normalized to that of OsACT. Bar = 1 mm. Data are means ± SE of three replicates and bars with different letters indicate significant differences at p < 0.05, as determined by ANOVA followed by the LSD test.
Next, GUS expression driven by the OsPIN1b promoter in root tip was evaluated (Fig. 5d). Compared with control conditions, pPIN1b::GUS expression in root tip was significantly reduced by SNP and GR24 treatments; however, the application of abamine ameliorated this effect. Moreover, under LN and LP conditions, the expression of pPIN1b::GUS was significantly upregulated by the application of cPTIO or abamine in comparison with that in the mock treatments, consistent with the gene expression results. Therefore, OsPIN1b is involved in the NO- and SL-induced elongation of seminal root under LN and LP conditions.
ospin1b mutants are less sensitive to LN and LP conditions, and SNP and GR24 treatments
The effect of LN and LP conditions on seminal root length of ospin1b-1 and ospin1b-2 and the WT (Dongjin) was assessed. Molecular characterisation revealed that T-DNAs were inserted into the promoter and 5′-URT regions, respectively, of the two ospin1b mutants. Compared with WT plants, OsPIN1b expression was almost completely suppressed in both ospin1b lines (Supplementary Fig. S4).
[3H]IAA transport and auxin concentration in the root tip, as well as the seminal root length of ospin1b mutants, were unaffected by LN, LP, SNP, and GR24 (Supplementary Fig. S5). Compared with WT plants, shorter seminal root length was recorded in two ospin1b mutants under different treatments. Compared with the control, the length of WT was increased under LN, LP, SNP, and GR24 treatments. However, the length of ospin1b mutants was not affected under control, LN, LP, SNP, or GR24 treatments (Fig. 6). These findings confirmed that OsPIN1b is involved in LN and LP in NO- and SL-mediated regulation of seminal root length.
Seminal root length in the wild type (WT, Dongjin) and ospin1b mutants. Seedlings were grown in hydroponic medium containing normal nutrition (control; 2.5 mM N, 300 μM ), low N, or low P (LN, 0.02 mM; LP, 2 µM P), or subjected to treatment with sodium nitroprusside (SNP, 10 µM) or GR24 (analog of SLs, 2.5 µM) for 14 days. (a), Morphology and (b,c) seminal root lengths of rice plants. Data are means ± SE of eight replicates (b,c) and bars with different letters indicate significant differences at p < 0.05, as determined by ANOVA followed by the LSD test.
LN and LP conditions, and SNP and GR24 application, do not influence pCYCB1;1::GUS expression in the ospin1b mutants
NO influences root elongation primarily by regulating root meristem activity43. In this study, the relative expression of OsCYCB1;1 was significantly decreased in d10 and d3 mutants relative to WT under control, LN, LP, and SNP treatments. However, application of GR24 markedly increased the levels of OsCYCB1;1 in d10, but not in d3 mutants, to the same extent as in WT (Fig. 7a).
OsCYCB1;1 gene and pCYCB1;1::GUS expression in rice seedlings. Seedlings were grown in hydroponic medium containing normal nutrition (control; 2.5 mM N, 300 μM P), low N, or low P (LN, 0.02 mM; LP, 2 µM), or subjected to treatment with sodium nitroprusside (SNP, 10 µM) or GR24 (analog of SLs, 2.5 µM) for 14 days. (a) The expression of OsCYCB1;1 gene in WT, d10 and d3 mutants. (b) pCYCB1;1::GUS expression in WT and ospin1b-1 mutant. Cell cycle activity of the root meristem, as monitored by the pCYCB1;1::GUS reporter, is shown. Bar = 500 μm. Data are means ± SE of three replicates (a) and bars with different letters indicate significant differences at p < 0.05, as determined by ANOVA followed by the LSD test.
ospin1b-1 transgenic plants expressing the pCYCB1;1::GUS construct were used to assess cell cycle activity in the root meristem (Fig. 7b). Compared to control conditions, pCYCB1;1::GUS expression in WT plants was greater under LN and LP conditions and following application of SNP and GR24. However, no significant change in pCYCB1;1::GUS expression was recorded in the root tip of the ospin1b-1 mutant. These findings suggested that OsPIN1b is involved in the role of NO and SLs in LN- and LP-induced root apical meristem activity.
Discussion
Strigolactones and strigolactone signaling are required for NO-mediated suppression of auxin transport to root apex
Auxin acts as a signaling intermediate in the response of root architecture to low phosphate supply45. An auxin transport-independent pathway is involved in the changes in primary roots induced by phosphate stress in Arabidopsis3,6,7,46,47. Studies in Arabidopsis have provided insight into the crosstalk between auxin and the root elongation response to P deficiency9,48. Moreover, auxin participates in the regulation of root elongation under N- and P-deficient conditions in rice12.
SLs, NO, and auxin transport are closely linked in the regulation of primary root elongation12,30. NO functions as a signaling molecule in the inhibition of auxin transport to the root apex and restrains primary root elongation in Arabidopsis30. In addition to NO, SLs may act as modulators of auxin transport to regulate root elongation12,32. In Arabidopsis, SLs modulate local auxin levels in a manner dependent on the auxin status of the plant32. In rice, the application of SLs markedly reduces auxin transport under N- and P-deficient conditions, which in turn increases seminal root length12.
In this study, compared with those in the control conditions, pDR5::GUS intensity, [3H]IAA activity, and IAA concentration in root tips were significantly reduced under LP and LN conditions (Supplementary Figs S1b,e and 2a), consistent with a previous report12. The application of SNP and GR24 under normal nutrition conditions and the application of cPTIO under low-nutrient conditions decreased and increased pDR5::GUS intensity and [3H]IAA activity in root tips, respectively, to levels similar to those under low-nutrient or control conditions (Supplementary Figs S1c,e and 2a). Furthermore, the application of NAA or NPA reversed the effect of SNP and cPTIO on seminal root length and pDR5::GUS expression (Supplementary Fig. S1). These results suggested that NO and SLs participate in LN- and LP-induced rice seminal root elongation by downregulating polar auxin transport from the shoot to the root tip.
We reported previously that SLs participate in LN- and LP-modulated seminal root elongation by inhibiting auxin transport12. In this study, pDR5::GUS expression, [3H]IAA transport, and OsPIN family expression were unaffected by SNP application, and OsPIN1b expression was unaffected by LN, LP, LN + cPTIO, and LP + cPTIO application, in the root tips of the d10 mutants (Figs 3 and 4; Supplementary Fig. S2). These results suggested that SL synthesis and signaling are required for the effect of NO on polar auxin transport from the shoot to root tip in rice.
OsPIN1b is involved in NO- and SL-induced root elongation in response to low N and P
The localisation of PINs in rice roots was reported by Wang et al.44: among rice 10 PIN genes, only OsPIN1b was expressed in the root cap; OsPIN1b/1c/5a/5b were expressed in the meristem; OsPIN1b/1c/9 were detected mainly in stele; OsPIN1a/1c/9/10a/10b were found in the LR cap region and OsPIN1c was also expressed in lateral root primordia (LRP); and OsPIN9 has higher activity in LRP and stele44. AtPIN1 was the first putative auxin efflux carrier to be characterised in Arabidopsis49. Subsequently, OsPIN1, ZmPIN1a, and ZmPIN1b were characterised in rice22 and maize50. Phylogenetic analysis showed that AtPIN1 is a highly conserved gene that may play a key role in polar auxin transport51. Gene structure analysis showed that rice has four OsPIN1 genes (OsPIN1a/1b/1c/1d), the distribution of each of which is similar to that of AtPIN144. AtPIN1 and OsPIN1b regulate root growth in different plant species22,30. Mutation of atpin1 decreased the length of primary root by influencing the root tip cell meristem30. ospin1b RNAi transgenic plants have fewer adventitious roots, but a similar root length, compared to WT plants22. However, we found that [3H]IAA transport and IAA concentration in root tips of ospin1b were unaffected by LN, LP, SNP, and GR24 (Supplementary Fig. S5), and seminal root length was shorter than that of WT plants under LN and LP conditions, and following SNP and GR24 application, which is inconsistent with a previous report22. This is likely due to our use of knockout T-DNA mutants while their RNAi transgenic plants are knockdown of OsPIN1b.
The influence of NO and SLs on auxin transport involves regulation of PIN proteins30,32. For example, the transcriptional and translational levels of AtPIN1 in root tip were reduced by the application of NO donors30. Strigolactones act by increasing the rate of AtPIN1 removal from the plasma membrane in shoot, but not in root52. OsPIN1a, 1b, 9, and 10a expression in rice root was significantly decreased under LN and LP conditions, and following GR24 application12. In this study, OsPIN1a-b, OsPIN2, and OsPIN10a-b expression in the WT was significantly reduced by SNP treatment compared with that in the control (Fig. 2b). Therefore, the expression of only OsPIN1a/1b/10a was downregulated under LN and LP conditions and following application of SNP and GR24. The expression of OsPIN2/10b was decreased under SNP treatment but not under LN and LP conditions, suggesting OsPIN2/10b was not involved in NO-mediated regulation of auxin transport under LN and LP. OsPIN1b was strongly expressed in root cap and meristem in transgenic rice plants with OsPIN-promoter-driven GUS44. Similarly, OsPIN1b expression was strongest in rice tips; OsPIN1b expression was at least 12- and 1,000-fold higher than that of OsPIN1a and OsPIN10a, respectively, in rice root tips (Supplementary Fig. S3). These findings suggested that OsPIN1b is involved in the role of LN and LP in the regulation of auxin transport by SL and NO.
The seminal root of ospin1b mutants was shorter than that of WT plants and root development in ospin1b mutants was less affected by low-nutrient conditions and application of SNP and GR24 (Fig. 6). Moreover, abamine relieved the inhibitory effect of SNP on OsPIN1b expression (Fig. 5); OsPIN1b expression was significantly upregulated by cPTIO application under LN and LP conditions to a level similar to that in the d10 mutant subjected to mock treatment (Fig. 5). These findings confirmed that OsPIN1b is involved in the role of LN and LP in SL- and NO-mediated elongation of seminal root by affecting root meristem activity (Fig. 7, Supplementary Fig. S6).
Methods
Plant materials and growth conditions
SL-deficient mutant (d10), SL signaling mutant (d3), and wild-type (WT, Shiokari) plants were provided by Shinjiro Yamaguchi of the Riken Plant Science Center. The T-DNA insertion ospin1b mutant lines (ospinib-1 and ospin1b-2) and the WT (cv. Dongjin) plants were obtained from the Rice Functional Genomics Express Database (RiceGE, Pohang City, South Korea).
Plants were grown in a greenhouse under natural light at day/night temperatures of 30/18 °C. Seven-day-old seedlings of uniform size and vigour were transplanted into holes in a lid placed over the top of pots (four holes per lid and three seedlings per hole). Nutrient solutions varying from one-quarter to full strength were applied for 1 week, followed by full-strength nutrient solution for a further week. Pots receiving normal nutrition (control) were filled with 2.5 mM N (NH4NO3) and 300 µM P (KH2PO4), and those receiving N- and P-deficient nutrition were filled with 0.02 mM N (LN) and 2 µM P (LP). To exclude any potential effects of potassium (K+) on the treatments, the low-P treatment solutions were supplemented with K+ to the same levels as those under sufficient P conditions (300 µM) using K2SO4. The full chemical composition of the International Rice Research Institute (IRRI) nutrient solution was (mM): 0.35 K2SO4, 1.0 CaCl2, 1.0 MgSO4·7H2O, 0.5 Na2SiO3; and (µM), 20.0 Fe-EDTA, 9.0 MnCl2, 0.39 (NH4)6Mo7O24, 20.0 H3BO3, 0.77 ZnSO4, and 0.32 CuSO4 (pH 5.5). The nutrient solution was replaced with fresh solution daily. Each treatment consisted of four replicates arranged in a completely randomised design to avoid edge effects. In addition, all experiments included three independent biological replicates.
Pharmacological treatments comprising 10 µM SNP (NO donor), 80 µM cPTIO (a NO scavenger), 10 nM NAA (an analogue of IAA), 2.5 µM GR24 (an analogue of SLs), and 100 µM abamine (SL synthesis inhibitor) were applied to the hydroponic media. Since NAA is more stable than IAA in nutrient solution, NAA was used as the analogue of IAA in this study. Localised application of NNPA (a polar auxin transport inhibitor) was performed by dispensing diluted agar containing 20 µM NPA directly from a pipette across the root-shoot junction23.
Measurement of root system architecture
Seminal roots were significantly longer than adventitious roots under our experimental conditions. Our preliminary experiment showed similar responses of seminal and adventitious roots to LN and LP treatments, and the number of adventitious roots did not change significantly under the different nutrient conditions during the experimental period12,43. Therefore, seminal roots were selected to study the effects of LN and LP on the rice root system. Seminal root lengths were measured using a ruler.
Determination of IAA
IAA concentrations in root tips were determined as described previously53. The fresh weight of samples was first determined, followed immediately by freezing in liquid N2. Measurement of free IAA by high-performance liquid chromatography (HPLC) was carried out as described previously53. An IAA standard was obtained from Sigma-Aldrich (St. Louis, MO, USA).
pDR5::GUS construct
To assess IAA distribution in rice plants, a pDR5::GUS construct was transformed into WT and d10 and d3 mutants using Agrobacterium tumefaciens (strain EHA105). The pDR5::GUS construct was provided by Professor Ping Wu’s group at Zhejiang University, Hangzhou, China. The samples used for IAA analysis were also used for histochemical GUS staining. The root tips were stained for GUS activity for 2 h at 37 °C. The stained tissues were photographed using an Olympus SZX2-ILLK stereomicroscope with a colour charge-coupled device (CCD) camera (Olympus, Tokyo, Japan)12.
pCYCB1;1::GUS construct
The pCYCB1;1::GUS fusion construct was transformed into rice plants. The pCYCB1; 1::GUS construct was provided by Professor Chuanzao Mao’s group at Zhejiang University, Hangzhou, China. The root tips were used for histochemical GUS staining, and were photographed using an SZX2-ILLK microscope with a colour CCD camera.
Measurement of NO in root tips
NO was imaged by diaminofluorescein-FM diacetate (DAF-FM DA) and epifluorescence microscopy. The root tips were loaded with 10 µM DAF-FM DA in 20 mM HEPES-NaOH buffer (pH 7.5). After incubating in the dark for 30 min, the root tips were washed three times in fresh buffer and immediately visualised using a stereomicroscope (Olympus MVX10) equipped with a colour CCD camera, with excitation and emission at 488 and 495–575 nm, respectively. Green fluorescence intensity was quantified using Photoshop software (Adobe Systems, San Jose, CA, USA)54. Data are presented as mean fluorescence intensities.
[3H]IAA transport
Shoot-to-root auxin transport in intact plants was assayed as described previously53. Polar transport of [3H]IAA was assayed using eight replicate root samples. The [3H]IAA solution contained 0.5 µM [3H]IAA (20 Ci mmol-1) in 2% DMSO, 25 mM MES (pH 5.2), and 0.25% agar.
Shoot-to-root auxin transport in intact plants was monitored as follows. [3H]IAA solution (20 µL) was applied to the cut surface after removal of rice shoots 2 cm above the root-shoot junction. The root tips were incubated in 4 mL of scintillation solution for 18 h (overnight) in the dark, and then sampled and weighed. [3H]IAA radioactivity was detected using a multipurpose scintillation counter (LS6500; Beckman-Coulter, Fullerton, CA, USA).
qRT-PCR
Total RNA was isolated from the root tips (0–0.5 cm) of rice seedlings. RNA extraction, reverse transcription, and qRT-PCR were performed as described previously23. Primer sets for the OsPIN genes are listed in Supplemental Table S1.
Data analysis
Data were pooled for calculation of the means and standard errors (SE) and subjected to one-way analysis of variance (ANOVA) followed by a least significant difference (LSD) test at P < 0.05 to determine the significance of differences between treatments. All statistical evaluations were conducted using SPSS software (ver. 11.0; SPSS Inc., Chicago, IL, USA).
References
Robertson, G. P. & Vitousek, P. Nitrogen in agriculture: balancing the cost of an essential resource. Annu Rev Environ Resour. 34, 97–125 (2009).
Gu, M., Chen, A., Sun, S. & Xu, G. Complex regulation of plant phosphate transporters and the gap between molecular mechanisms and practical application: What is missing? Mol Plant. 9(3), 396–416 (2016).
López-Bucio, J., Cruz-Ramírez, A. & Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol. 6, 280–287 (2003).
Chun, L., Mi, G., Li, J., Chen, F. & Zhang, F. Genetic analysis of maize root characteristics in response to low nitrogen stress. Plant Soil. 276, 369–382 (2005).
Gruber, B., Giehl, R., Friedel, S. & Wirén, N. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol. 163, 161–179 (2013).
Williamson, L., Ribrioux, S., Fitter, A. & Leyser, H. Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol. 126, 875–882 (2001).
Linkohr, B., Williamson, L., Fitter, A. & Leyser, H. Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. Plant J. 29, 751–760 (2002).
Chevalier, F., Pata, M., Nacry, P., Doumas, P. & Rossignol, M. Effects of phosphate availability on the root system architecture: large-scale analysis of the natural variation between Arabidopsis accessions. Plant Cell Environ. 26, 1839–1850 (2003).
Pérez-Torres, C. et al. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell. 20, 3258–3272 (2008).
Zheng, L. et al. Physiological and transcriptome analysis of iron and phosphorus interaction in rice seedlings. Plant Physiol. 151, 262–274 (2009).
Rai, V. et al. Iron availability affects phosphate deficiency-mediated responses, and evidence of cross-talk with auxin and Zinc in Arabidopsis. Plant cell Physiol. 56(6), 1107–1123 (2015).
Sun, H. et al. Strigolactones are involved in phosphate and nitrate deficiency-induced root development and auxin transport in rice. J Exp Bot. 65, 6735–6746 (2014).
Tian, Q., Chen, F., Liu, J., Zhang, F. & Mi, G. Inhibition of maize root growth by high nitrate supply is correlated to reduced IAA levels in roots. J Plant Physiol. 165, 942–951 (2008).
Zhang, J., Xu, L., Wang, F., Deng, M. & Yi, K. Modulating the root elongation by phosphate/nitrogen starvation in an OsGLU3 dependant way in rice. Plant Signal Behav. 7, 1144–1145 (2012).
Casimiro, I. et al. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell. 13, 843–852 (2001).
Benkova, E. et al. Local, efflux dependent auxin gradients as a common module for plant organ formation. Cell. 115, 591–602 (2003).
Casimiro, I. et al. Dissecting Arabidopsis lateral root development. Trends Plant Sci. 8, 165–171 (2003).
Vieten, A., Sauer, M., Brewer, P. & Friml, J. Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci. 12(4), 160–168 (2007).
Petrásek, J. & Friml, J. Auxin transport routes in plant development. Development. 136(16), 2675–2688 (2009).
Peret, B. et al. AUX/LAX genes encode a family of auxin influx transporters that perform distinct functions during Arabidopsis development. Plant Cell. 24, 2874–2885 (2012).
Friml, J. Auxin transport-shaping the plant. Curr Opin Plant Biol. 6, 7–12 (2003).
Xu, M., Zhu, L., Shou, H. & Wu, P. A PIN1 family gene, OsPIN1, involved in auxin-dependent adventitious root emergence and tillering in rice. Plant Cell Physiol. 46, 1674–1681 (2005).
Chen, Y., Fan, X., Song, W., Zhang, Y. & Xu, G. Over-expression of OsPIN2 leads to increased tiller numbers, angle and shorter plant height through suppression of OsLAZY1. Plant Biotech J. 10, 139–149 (2012).
Zhang, Q. et al. The putative auxin efflux carrier OsPIN3t is involved in the drought stress response and drought tolerance. Plant J. 72, 805–816 (2012).
Lu, G. et al. OsPIN5b modulates rice (Oryza sativa) plant architecture and yield by changing auxin homeostasis, transport and distribution. Plant J. 83(5), 913–925 (2015).
Pagnussat, G., Lanteri, M. & Lamattina, L. Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol. 132, 1241–1248 (2003).
Correa-Aragunde, N., Graziano, M. & Lamattina, L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta. 218, 900–905 (2004).
Lombardo, M., Graziano, M., Polacco, J. & Lamattina, L. Nitric oxide functions as a positive regulator of root hair development. Plant Signal Behav. 1(1), 28–33 (2006).
Zhao, D., Tian, Q., Li, L. & Zhang, W. Nitric oxide is involved in nitrate-induced inhibition of root elongation in Zea mays. Ann Bot. 100, 497–503 (2007).
Fernández-Marcos, M., Sanz, L., Lewis, D., Muday, G. & Lorenzo, O. Nitric oxide causes root apical meristem defects and growth inhibition while reducing PIN-FORMED 1 (PIN1)-dependent acropetal auxin transport. Proc Natl Acad Sci USA 108, 18506–185011 (2011).
Kapulnik, Y. et al. Strigolactones interact with ethylene and auxin in regulating root-hair elongation in Arabidopsis. J Exp Bot. 62, 2915–2924 (2011).
Ruyter-Spira, C. et al. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: another belowground role for strigolactones? Plant Physiol. 155, 721–734 (2011).
Koltai, H. Strigolactones are regulators of root development. New Phytol. 190, 545–549 (2011).
Arite, T., Kameoka, H. & Kyozuka, J. Strigolactone positively controls crown root elongation in rice. J Plant Growth Regul. 31, 165–172 (2012).
Mayzlish-Gati, E. et al. Strigolactones are involved in root response to low phosphate conditions in Arabidopsis. Plant Physiol. 160, 1329–1341 (2012).
Rasmussen, A. et al. Strigolactones suppress adventitious rooting in Arabidopsis and Pea. Plant Physiol. 158, 1976–1987 (2012).
Bai, S. et al. PIF3 is involved in the primary root growth inhibition of Arabidopsis induced by nitric oxide in the light. Mol Plant. 4, 616–625 (2014).
Manoli, A. et al. NO homeostasis is a key regulator of early nitrate perception and root elongation in maize. J Exp Bot. 65, 185–200 (2014).
Waldie, T., McCulloch, H. & Leyser, O. Strigolactones and the control of plant development: lessons from shoot branching. Plant J. 79, 607–622 (2014).
De Cuyper, C. et al. From lateral density to nodule number, the strigolactone analogue GR24 shapes the root architecture of medicago truncatula. J Exp Bot. 66, 137–146 (2015).
Sun, H. et al. Nitric oxide generated by nitrate reductase increases nitrogen uptake capacity by modulating lateral root formation and inorganic nitrogen uptake rate in rice. J Exp Bot. 66(9), 2449–2459 (2015a).
Sun, H. et al. A strigolactones signal is required for adventitious root formation in rice. Ann Bot. 115(7), 1155–1162 (2015b).
Sun, H. et al. Strigolactones are required for nitric oxide to induce root elongation in response to nitrogen and phosphate deficiencies in rice. Plant Cell Environ. 39(7), 1473–1484 (2016).
Wang, J. et al. Expression of PIN genes in rice (Oryza sativa L.): tissue specificity and regulation by hormones. Mol Plant. 2, 823–831 (2009).
Niu, Y. et al. Responses of root architecture development to low phosphorus availability: a review. Ann Bot. 112, 391–408 (2013).
Ticconi, C., Delatorre, C., Lahner, B., Salt, D. & Abel, S. Arabidopsis pdr2 reveals a phosphate-sensitive checkpoint in root development. Plant J. 37, 801–814 (2004).
Jain, A. et al. Variations in the composition of gelling agents affect morphophysiological and molecular responses to deficiencies of phosphate and other nutrients. Plant Physiol. 150, 1033–1049 (2009).
Chiou, T. & Lin, S. Signaling Network in Sensing Phosphate Availability in Plants. Annu Rev Plant Biol. 62, 185–206 (2011).
Okada, K., Ueda, J., Komaki, M., Bell, C. & Shimura, Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell. 3, 677–684 (1991).
Carraro, N., Forestan, C., Canova, S., Traas, J. & Varotto, S. ZmPIN1a & ZmPIN1b encode two novel putative candidates for polar auxin transport and plant architecture determination of maize. Plant Physiol. 142, 254–264 (2006).
Friml, J. & Palme, K. Polar auxin transport-old questions and new concepts? Plant Mol Biol. 49, 273–284 (2002).
Shinohara, N., Taylor, C. & Leyser, O. Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biol. 11(1), e1001474 (2013).
Song, W. et al. Auxin distribution is differentially affected by nitrate in roots of two rice cultivars differing in responsiveness to nitrogen nutrients. Ann of Bot. 112, 1383–1393 (2013).
Guo, F. & Crawford, N. Arabidopsis nitric oxide synthase1 is targeted to mitochondria and protects against oxidative damage and dark-induced senescence. Plant Cell. 17, 3436–3450 (2005).
Acknowledgements
This work was funded by the National Nature Science Foundation of China (grant no. 31672225, 31471936, 31601821 and 31401937). The Province Nature Science Foundation of Jiangsu (grant no. 20140694). Innovative Research Team Development Plan of the Ministry of Education of China (No. IRT1256), the 111 Project (No. 12009), the Priority Academic Program Development of Jiangsu Higher Education Institutions Project, China Scholarship Council (CSC). The d10 and d3 mutants were provided by Shinjiro Yamaguchi of RIKEN Plant Science Center, Japan. The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/Ihwjlk.
Author information
Authors and Affiliations
Contributions
Y.L.Z., H.W.S. and J.Y.T. designed the experiments and wrote the manuscript. H.W.S. and J.Y.T., Y.B. and M.M.H. conducted the measurements, data analysis. J.J.L., X.N.C., X.H.Z. and L.L. assisted with the experiment. X.N.X., K.Y., Q.Z.Z. and G.H.X. assisted with the manuscript. These authors reviewed the manuscript before the submission.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, H., Tao, J., Bi, Y. et al. OsPIN1b is Involved in Rice Seminal Root Elongation by Regulating Root Apical Meristem Activity in Response to Low Nitrogen and Phosphate. Sci Rep 8, 13014 (2018). https://doi.org/10.1038/s41598-018-29784-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-29784-x
This article is cited by
-
Auxin efflux carrier ZmPIN1a modulates auxin reallocation involved in nitrate-mediated root formation
BMC Plant Biology (2023)
-
Auxin regulation on crop: from mechanisms to opportunities in soybean breeding
Molecular Breeding (2023)
-
Phosphorus Scavenging and Remobilization from Root Cell Walls Under Combined Nitrogen and Phosphorus Stress is Regulated by Phytohormones and Nitric Oxide Cross-Talk in Wheat
Journal of Plant Growth Regulation (2023)
-
Tissue specificity and responses to abiotic stresses and hormones of PIN genes in rice
Biologia (2022)
-
Proteomics of mercury-induced responses and resilience in plants: a review
Environmental Chemistry Letters (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.