Abstract
No international consensus regarding the resection of the para-aortic lymph node (PALN) station Ln16b1 during pancreatoduodenectomy for pancreatic ductal adenocarcinoma (PDAC) has been reached. The present retrospectively investigated 264 patients with PDAC who underwent curative pancreatoduodenectomy or total pancreatectomy between 2005–2015. In 95 cases, the PALN were separately labelled and histopathologically analysed. Metastatic PALN (PALN+) were found in 14.7% (14/95). PALN+ stage was associated with increased regional lymph node metastasis. The median overall survival (OS) of patients with metastatic PALN and with non-metastatic PALN (PALN−) was 14.1 and 20.2 months, respectively. Five of the PALN+ patients (36%) survived >19 months. The OS of PALN+ and those staged pN1 PALN− was not significantly different (P = 0.743). Patients who underwent surgical exploration or palliative surgery (n = 194) had a lower median survival of 8.8 (95% confidence interval: 7.3–10.1) months. PALN status could not be reliably predicted by preoperative computed tomography. We concluded that the survival data of PALN+ cases is comparable with advanced pN+ stages; one-third of the patients may expect longer survival after radical resection. Therefore, routine refusal of curative resection in the case of PALN metastasis is not indicated.
Similar content being viewed by others
Introduction
One of the unsolved difficulties in treating patients with pancreatic ductal adenocarcinoma (PDAC) is early lymph node spread, which probably leads to tumour recurrence even after complete surgical resection. Consequently, extended lymphadenectomy during pancreatoduodenectomy for PDAC has been studied in multiple trials, which have failed to show results of longer survival1, 2. Recently, an International Study Group for Pancreatic Surgery (ISGPS) consensus statement defined the international standard lymphadenectomy based on a current literature review3. However, no consensus was reached for recommending routine resection of the para-aortic lymph node (PALN) station Ln16b1 dorsal to the pancreas. Metastasis to this lymph node station is classified as pM1 stage, because these lymph nodes do not belong to the regional lymph node stations. Several studies concluded that PALN metastases are associated with poor survival, but the effect of Ln16b1 metastasis and its resection on survival remained unclear. An updated meta-analysis recently recapitulated the prognostic impact of PALN metastasis and encouraged intraoperative assessment of PALN status. Nevertheless, a clear recommendation whether to complete or to avoid tumour resection in the case of PALN metastasis was not given4. In fact, single studies advocated against performing tumour resection if PALN metastasis is histologically verified, thus making PALN metastasis a watershed for curative resection5. These contradictive management recommendations warrant further data acquisition and analysis. Almost all available data investigating the impact of PALN resection emanate from Asian – most frequently Japanese – centres. The present study provides additional evidence and aims to assess the prognosis of PALN metastases and their resection in a European population, with a special focus on regional lymph node status.
Results
Description of patient, histopathology and morbidity variables
Some 264 patients underwent pancreatoduodenectomy or total pancreatectomy for PDAC within the study period. In 95 cases, the resected PALN were separately labelled and histopathologically analysed. The median follow-up time was 17.9 months. Metastatic PALN (PALN+) were found in 14.7% (14/95) and a mean number of 6 PALN were resected. Most frequently, a pylorus-preserving pancreatoduodenectomy (PPPD) was performed (87%) and most standard patient and surgical characteristics were not significantly different between cases with metastatic and non-metastatic PALN (PALN−) (Table 1). However, the percentage of locally advanced tumours was higher in the PALN+ subgroup, and there was a trend that the PALN+ patients had higher preoperative serum CA 19-9 levels. Moreover, the 14 PALN+ cases had a significant higher rate of regional lymph node spread (N1, 100%; P = 0.002) and a higher lymph node ratio (LNR), reflecting an advanced lymphovascular propagation (Table 2). In the majority of the 14 PALN+ cases, only one PALN was affected (8/14; range of metastatic PALN: 1–7). Postoperative morbidity was equal in both subgroups, except for a significant higher occurrence of postoperative diarrhoea in the PALN+ subgroup. A postoperative pancreatic fistula (POPF) occurred in 21% (PALN+) and 14% (PALN−) (Table 3). The overall 30-day mortality was 2.1% (2/95).
Correlation of PALN status with survival
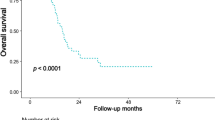
The median overall/ disease-free survival (DFS) rates of patients with metastatic PALN and with non-metastatic PALN was 14.1 / 7.4 months and 20.2 (95% confidence interval [CI]: 17.0–24.8)/ 12.3 (95% CI 10.0–16.0) months, respectively (Fig. 1). On univariate analysis, PALN status did not significantly correlate with the different overall survival data (P = 0.093). However, PALN status (P = 0.03) and failure to receive adjuvant treatment (P < 0.001) were the only significant factors associated with a shorter DFS on multivariate analysis. To compare para-aortic with regional lymph node metastases, we further stratified cases with non-metastatic PALN according to the pN-stage. The PALN− subgroup included 37 cases staged N0 and 44 cases with regional lymph node metastasis (N1). The median overall survival of the N0 subgroup was 23.9 (95% CI 18.8–48.0) months and significantly longer (P = 0.047), compared with N1 (median survival 19.3 months, 95% CI 10.9–22.9) or PALN+ cases (Fig. 1B). The overall survival of patients with PALN+ and those staged PALN− N1 was not significantly different (P = 0.743). Likewise, the PALN− N0 subgroup had a significant longer DFS (P = 0.013, Fig. 1C).
Overall and disease-free survival of patients with para-aortic lymph node resection. (A) Overall survival of patients with para-aortic lymph node (PALN) resection (n = 95). Patient subgroups with PALN metastasis (PALN+, n = 14) and without PALN metastasis (PALN−, n = 81) were plotted. (B,C) Overall survival (B) and disease-free survival (C) of patients with PALN resection separated into PALN+ (n = 14), PALN− pN0 (n = 37), and PALN− pN1 (n = 44) subgroups according to the regional lymph node status. (D) Overall survival of PALN + patients (n = 14) compared with patients after palliative surgery for unresectable or metastastic pancreatic cancer (n = 194).
The new (8th edition) classification of TNM staging of the Union for International Cancer Control (UICC) introduced a N2 stage for PDAC (≥4 regional lymph node metastases) because recent studies had underlined that the number of lymph node metastases significantly affected survival6, 7. When we retrospectively applied the 8th edition nodal staging system to the present cohort of patients, the median survival of the patients staged pN0, pN1, and pN2 was 23.9 months, 19.3 months, and 17.0 months, respectively. The pN2 subgroup (n = 24) included 8 patients with PALN metastases, and exclusion of these cases resulted in a median survival of 10.9 months (95% CI 9.4–28.0). In contrast, patients with 1–3 regional lymph node metastases (pN1 according to the 8th edition of TNM staging) and no PALN metastases (n = 28) had a median survival of 19.5 months (95% CI 15.4–25.0). Thus, the survival prognosis of the analysed PALN+ patients was in-between the prognosis of pN1 and pN2 staged cases.
To compare the survival probability of patients with metastatic PALN and palliatively treated patients, we further identified all patients who underwent surgical exploration or palliative bypass surgery for unresectable or metastatic PDAC within the study period. These 194 patients had a median survival of 8.8 (95% CI 7.3–10.1) months, which was not significantly shorter compared with the PALN+ subgroup (Fig. 1D). However, some patients with metastatic PALN demonstrated a long-term survival, not seen in the palliative setting: 5 patients (36%) with positive PALN survived more than 19 months (range 19.6–57.1), mirroring the median outcome of patients treated in curative intent.
Preoperative prediction of PALN status using computed tomography
Next, it was assessed whether the presence of metastatic PALN could be predicted based on the preoperative CT-imaging studies. The preoperative CT studies were available in only 10 of the 14 patients with positive PALN status. The 10 cases with PALN+ status and available preoperative CT studies were then matched according to age, sex, and tumour stage with 10 PALN− cases (and available preoperative CT studies), and analysed by an experienced radiologist, who was blinded with respect to the histopathological outcome. Positive PALN status was radiologically suspected in only 2 of the 10 cases with PALN metastases (sensitivity: 20%, specificity: 100%) (Table 4). Interestingly, the 2 cases had a high number of tumour-involved PALN (3 and 7), whereas the mean number of metastatic PALN was 1 in non-suspected (based on CT imaging) PALN (Table 4). 18F-Fluorodeoxyglucose positron emission/computed tomography (FDG/PET-CT) scans were not performed routinely in the preoperative setting of patients with PDAC. A preoperative FDG/PET-CT was available for only 1 patient with PALN metastases, but the uptake values did not predict the PALN involvement in this case.
Discussion
The discussion of extended lymphadenectomy or PALN resection during PDAC resection started years ago, but is still an ongoing and controversial debate as demonstrated by the very recent literature. Consequently, no consensus has been reached on the management of PALN3. The present study and recent meta-analyses congruently demonstrate that approximately 14–18% of PDAC of the pancreatic head harbour PALN metastases at the time of resection4, 8, and that this subgroup of patients has a relative poor prognosis with a median overall survival of 13–14 months. Recently, Hackert et al. published an overall median survival of 12.3 months after resection of synchronous oligometastatic PDAC (either liver or interaortocaval lymph node metastases)9. Taken together, these data further support that the Asian and European outcome data after PALN resection are comparable. These include the quality of PALN resection, indicated by the mean number of resected PALN: 6 (present study) compared with 4.310 and 5.011 in the Asian studies. Interestingly, our analysis showed that one-third of the patients with PALN metastases survived 19 months or longer after resection. This is in line with results from the largest multicentre study to date, which reported that 26 out of 102 patients with metastatic PALN survived for more than 2 years10. There are even reports of long-term survival after removal of PALN metastases12. When we compared the median survival duration of patients with palliative surgery (8.8 months) or PALN+ resection (14.1 months), patients in the latter subgroup demonstrated a longer survival of approximately 5 months, thus, supporting the resection of PALN (Ln16b1) at least in subgroups of patients. Based on the fact that PALN metastases are associated with early tumour recurrence, others have recommended that intraoperative sampling of PALN with frozen section analysis should be routinely performed, and consequently, that tumour resection in the case of such lymph node metastases should be avoided5. In contrast, we do not conclude that PALN metastases should be considered a general contraindication for curative resection or multimodal strategies, because of the lack of superior treatment alternatives. Moreover, the present data revealed that most frequently only one single lymph node of the Ln16b1 group was tumour infiltrated, which limits the prediction of intraoperative frozen section of single nodes. In future, the possibility of genetic subtype analysis may further define patients who would benefit from PALN resection13.
The survival and DFS difference of patients with PALN metastases and regional pN1pM0 stage is marginal, and PALN+ cases are associated with a high burden of lymphovascular tumour spread. The survival prognosis is comparable to the new N2 stage of the UICC 8th edition staging system, which should be evaluated in larger series. Therefore, PALN metastases may characterise a subgroup of PDAC with extensive lymph node metastases, which might benefit additionally from neoadjuvant treatment. The data further open the discussion whether PALN should be classified as regional lymph nodes for PDAC.
However, the preoperative prediction of PALN status is difficult, if not impossible14. Imai et al. could not detect PALN metastasis in any of their patients by preoperative imaging studies15. In the present study, only 2 cases with extensive PALN metastasis (3 and 7 Ln16b1 nodes infiltrated) were identified by the preoperative CT scans. Thus, in most cases the sensitivity of CT imaging is insufficient for prediction of PALN metastases. Other imaging modalities such as FDG/PET-CT or endoscopic ultrasound (EUS) have been investigated in staging of PDAC. Whereas FDG/PET-CT may be more sensitive in the diagnosis of primary PDAC and distant metastases compared with contrast-enhanced CT, its accuracy for detection of local nodal tumor metastases is poor and was reported between 37.5 and 42%16, 17. The accuracy of EUS for staging of nodal involvement reached 65% or even higher in single studies, but a meta-analysis demonstrated its general limits in nodal staging based on the pooled accuracy parameters18, 19. In summary, the accuracy of CT, FDG/PET-CT or EUS is insufficient to support selective treatment approaches, e.g. neoadjuvant therapy in case of FDG/PET-CT positive PALN.
The present study has some limitations that should be considered. Because PALN resection was routinely performed at our institution, there was no comparison of the morbidity of cases with and without PALN resection. One of the most relevant complications is the development of a POPF, which was diagnosed in 14.7% of all cases. We do not think that dissection of the PALN generally increases the risk for POPF because it does not affect the pancreatic neck dissection or the site of pancreatojejunostomy. A definite allocation of the resected lymph nodes to the Ln16b1 station (PALN) was only possible in 95 of the 264 cases, because PALN were not labelled routinely at the time of resection, which limited the available cases. Further, the low number of cases included for analysis of CT prediction of PALN status is insufficient to calculate valid test statistics, but was predetermined by the 10 available CT studies of patients with PALN metastases.
In conclusion, the present study confirms that patients with PALN metastases (pM1 LYM) have a relative poor prognosis, resulting in a median survival of approximately 14 months. However, one-third of these patients demonstrated a longer survival. Compared with other studies on PALN resection, the present study explicitly analysed the lymph node status and demonstrated that the survival of patients with positive PALN was comparable to patients with pN1pM0 disease, and even more to pN2pM0 patients according to the 8th edition of TNM staging (UICC). Because preoperative imaging studies fail to reliably predict PALN metastases, PALN status prior to tumour resection can only be assessed by intraoperative frozen section analysis. However, abandoning tumour resection in the case of Ln16b1 metastases should not be routinely recommended; we would rather recommend a tailored approach taking into account the individual risk profile of the patient.
Methods
Study design and patients
Data were obtained from a prospective database and retrospectively analysed. All patients who underwent partial pylorus-preserving PD (PPPD), classic PD (cPD), or total pancreatectomy (TP) for PDAC of the pancreatic head or neck between January 2005 and March 2015 at the Department of Visceral, Thoracic and Vascular Surgery, University Hospital Carl Gustav Carus, Technische Universität Dresden, Germany, were screened for inclusion. Although PALN resection was performed routinely, the resected lymph nodes could not be unequivocally allocated to the PALN in all cases during pathological workup. Only cases with unequivocally labelled PALN were included for further analysis. The patients were divided into two subgroups: patients with a PALN-positive status (histopathological tumour invasion of at least one PALN, PALN+) and patients without PALN metastasis (PALN−). For survival analysis, PALN-negative cases were subdivided according to the pN stage. Patients who underwent palliative surgery for unresectable or metastatic PDAC in the same observation period were likewise considered for comparative survival analysis.
Tumour recurrence was assessed by regular follow-up examinations in the outpatient clinic, telephone interviews, or by contacting the respective primary physician of the patients. Tumour progression was assumed, if imaging modalities, e.g. sonography, computed tomography (CT) or magnetic resonance imaging (MRI) scans, or the clinical and laboratory examination, e.g. carbohydrate angigen 19-9 (CA 19-9) were indicative. Borderline resectability of the tumours was assessed using the National Comprehensive Cancer Network (NCCN) definition based on the preoperative CT scans20. The experimental protocol of the study was approved by the local Ethics Committee of the TU Dresden (decision number EK70022017). All methods were carried out in accordance with relevant guidelines and informed consent was obtained from the all included patients.
Operative technique
The basic operative technique of the PPPD and cPD was recently described21. After an extended Kocher manoeuvre, the PALN were dissected on the ventral aspect and between the vena cava and aorta, beginning at the right side of the coeliac trunk and origin of the superior mesenteric artery to the origin of the inferior mesenteric artery.
Pathological assessment
Para-aortic lymph node samples were examined macroscopically, dissected and all palpable nodes were submitted for histological examination. Histological analysis was carried out using haematoxylin & eosin slides without serial sectioning or immunohistochemical analyses for single tumour cells. A metastasis was diagnosed when tumour infiltration with desmoplastic reaction was visible.
Radiological assessment and patient matching
Radiological prediction of postoperative, histopathological PALN tumour invasion was investigated using preoperative CT imaging studies. Therefore, the 14 patients with PALN metastases were matched 1:1 to the patients within the PALN− subgroup, taking into consideration the following variables: age, body mass index (BMI), TNM stage, type of operation. An experienced radiologist, who was blinded with respect to the histopathological outcome, retrospectively analysed the available preoperative CT studies of the cases for assessment of PALN status. The para-aortic anatomical region of the Ln16b1 station was examined on venous-phase sequences. Lymph nodes >1 cm and those showing an inhomogeneous structure and/or irregular borders were considered suspicious for tumour invasion.
Statistical analysis
The R software package (R version 3.1.3, The R Foundation for Statistical Computing; http:\\www.R-project.org\) was used for statistical calculation and obtaining data plots. The significance level for all calculations was set at P = 0.05. The Fisher-Exact test and the unpaired t test were used to test categorical or quantitative variables. Uni- and multivariate analyses were computed by using the Cox proportional hazards models. The following variables were considered for univariate analysis: patient age >70 years, neo- and adjuvant treatment, T3/4 versus T1/2 tumours, N1 versus N0, R1 versus R0, G3/4 versus G1/2, and PALN+ versus PALN−. The lymph node ratio (LNR) was calculated as the quotient between tumour infiltrated lymph nodes and resected lymph nodes, and was stratified in LNR <0.2 and ≥0.2. Significant variables on univariate analysis were entered into the multivariable test. The Kaplan–Meier method was used for overall and disease-free survival (DFS) curves, and the log-rank test was used to identify differences between the survival curves. OS was computed by the date of death or the time of last contact (censored), and DFS was defined as the time between the index operation and the last follow-up contact without disease progression. The follow-up time was defined from surgery to death, or the last patient contact.
References
Michalski, C. W. et al. Systematic review and meta-analysis of standard and extended lymphadenectomy in pancreaticoduodenectomy for pancreatic cancer. The British journal of surgery 94, 265–273 (2007).
Nimura, Y. et al. Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas: long-term results of a Japanese multicenter randomized controlled trial. Journal of hepato-biliary-pancreatic sciences 19, 230–241 (2012).
Tol, J. A. et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 156, 591–600 (2014).
Paiella, S. et al. The prognostic impact of para-aortic lymph node metastasis in pancreatic cancer: A systematic review and meta-analysis. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 42, 616–624 (2016).
Schwarz, L. et al. Para-aortic lymph node sampling in pancreatic head adenocarcinoma. The British journal of surgery 101, 530–538 (2014).
Strobel, O. et al. Pancreatic adenocarcinoma: number of positive nodes allows to distinguish several N categories. Annals of surgery 261, 961–969 (2015).
Allen, P. J. et al. Multi-institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients With Pancreatic Adenocarcinoma. Annals of surgery 265, 185–191 (2017).
Agalianos, C., Gouvas, N., Papaparaskeva, K. & Dervenis, C. Positive para-aortic lymph nodes following pancreatectomy for pancreatic cancer. Systematic review and meta-analysis of impact on short term survival and association with clinicopathologic features. HPB: the official journal of the International Hepato Pancreato Biliary Association 18, 633–641 (2016).
Hackert, T. et al. Radical surgery of oligometastatic pancreatic cancer. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 43, 358–363 (2017).
Sho, M. et al. Postoperative prognosis of pancreatic cancer with para-aortic lymph node metastasis: a multicenter study on 822 patients. Journal of gastroenterology 50, 694–702 (2015).
Murakami, Y. et al. Prognostic impact of para-aortic lymph node metastasis in pancreatic ductal adenocarcinoma. World journal of surgery 34, 1900–1907 (2010).
Masui, T. et al. Long-term survival after resection of pancreatic ductal adenocarcinoma with para-aortic lymph node metastasis: case report. World journal of surgical oncology 11, 195 (2013).
Bailey, P. et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531, 47–52 (2016).
McDermott, S. et al. Accurate prediction of nodal status in preoperative patients with pancreatic ductal adenocarcinoma using next-gen nanoparticle. Translational oncology 6, 670–675 (2013).
Imai, H. et al. Preoperative assessment of para-aortic lymph node metastasis in patients with pancreatic cancer. International journal of clinical oncology 15, 294–300 (2010).
Kauhanen, S. P. et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Annals of surgery 250, 957–963 (2009).
Asagi, A. et al. Utility of contrast-enhanced FDG-PET/CT in the clinical management of pancreatic cancer: impact on diagnosis, staging, evaluation of treatment response, and detection of recurrence. Pancreas 42, 11–19 (2013).
Li, J. H. et al. Endoscopic ultrasonography for tumor node staging and vascular invasion in pancreatic cancer: a meta-analysis. Digestive surgery 31, 297–305 (2014).
Soriano, A. et al. Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. The American journal of gastroenterology 99, 492–501 (2004).
Bockhorn, M. et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 155, 977–988 (2014).
Welsch, T., Bork, U., Distler, M. & Weitz, J. Top-down approach to the superior mesenteric artery and the mesopancreas during pancreatoduodenectomy for pancreatic cancer. Journal of surgical oncology 113, 668–671 (2016).
Acknowledgements
The present work was not supported by any third party funding or research grant.
Author information
Authors and Affiliations
Contributions
S.H. performed most of the data acquisition and analysis, and drafted the manuscript. V.P. performed the radiologic assessment and analysis. F.M. and M.D. contributed to the data collection and reviewed the manuscript. D.E.A. was responsible for the pathological analysis and reporting. H.D.S. helped with the conceptional design and critically reviewed the manuscript. J.W. and T.W. designed the study, contributed to the statistical analysis, data presentation and finalized the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hempel, S., Plodeck, V., Mierke, F. et al. Para-aortic lymph node metastases in pancreatic cancer should not be considered a watershed for curative resection. Sci Rep 7, 7688 (2017). https://doi.org/10.1038/s41598-017-08165-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-08165-w
This article is cited by
-
Impact of para aortic lymph node removal on survival following resection for pancreatic adenocarcinoma
BMC Surgery (2023)
-
Pancreaticoduodenectomy with Para-aortic Lymph Node Dissection for Periampullary Cancer
Indian Journal of Surgical Oncology (2023)
-
Impact of resection margin status on survival in advanced N stage pancreatic cancer – a multi-institutional analysis
Langenbeck's Archives of Surgery (2021)
-
Prognostic impact of para-aortic lymph node metastases in non-pancreatic periampullary cancer
World Journal of Surgical Oncology (2020)
-
Therapeutic response assessment in pancreatic ductal adenocarcinoma: society of abdominal radiology review paper on the role of morphological and functional imaging techniques
Abdominal Radiology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.