Abstract
Embryonic cryopreservation has a relatively low survival rate because of cytoskeletal damage. However, molecular anti-freezing mechanisms have been largely unexplored. This study investigated the significance of RhoA, involved in embryonic development, and the Rho/RhoA-associated kinase (ROCK) signalling pathway in cryopreservation. The anti-freezing mechanism in murine dormant embryos, compared with normal blastocysts, was assessed by combining molecular, physiological and pharmacological approaches. Real-time PCR and western blotting experiments showed high RhoA expression in cryo-dormant and dormant embryos. RhoA GTPases were overexpressed on the surface of trophectoderm cells in dormant embryos. Treatment with Y-27632, a ROCK antagonist, decreased survival of both normal and dormant blastocysts, while recombinant RhoA protein remarkably increased survival, after freeze–thawing, of normal hatched blastocysts. Our findings elucidated the molecular mechanism of anti-freezing, involving RhoA phosphorylation, meditated by the Rho/ROCK signalling pathway, in hatched and diapaused murine blastocysts. In addition, evidence for a potentially protective additive suggests a new method for improving the anti-freezing potential of mammalian embryos, without protecting the zona pellucida.
Similar content being viewed by others
Introduction
It is widely known that mammalian delayed implantation is of great importance in reproductive biology. During entry and maintenance of diapause, cell cycle progression is arrested by p21 protein family and is restrained at G11. For instance, in mice and rats, ovariectomy before the presumed oestrogen surge, on the morning of day 4 of pregnancy, results in failure of implantation and initiates a dormancy state in blastocysts2.
Previous studies showed that dormant mouse embryos have higher survival rates than normal embryos after cryopreservation3. Generally, expansion ability after freezing-thawing procedure is reckoned as an indicator of embryonic survival status4. Although cryopreservation of mammalian oocytes is a routine technique used worldwide, it is still crucial to decrease cryo-damage of cells during cryopreservation steps for efficient survival of embryos or oocytes. Cryo-injury influences embryonic development, even interfering with the cytokinesis and receptor-mediated signal transduction5,6,7,8.
RhoA is a small guanosine triphosphatase (GTPase) protein from the Rho family and it belongs to Ras homologue gene family. RhoA was reported to be involved in complex cellular processes, including cell motility, cell adhesion and chromosome inheritance9. RhoA is a Rho family regulator of cytokinesis in dividing embryos10, whereas Rho-associated protein kinase (ROCK) is a key downstream effector of RhoA. It was reported that the embryonic development is dependent on RhoA in Xenopus and Drosophila melanogaster 11,12,13. In addition, blocking of ROCK prevented early cleavage development in early zebrafish and mouse embryos14. RhoA also plays a pivotal role in G1 cell cycle progression, primarily by regulating expression of cyclin D1 and cyclin-dependent kinase inhibitors (p21 and p27). On one hand, RhoA facilitates the entry into S phase by degradation of the cyclin-dependent kinase inhibitor p27kip1 15. On the other hand, it suppresses p21 levels that block G1. As a binary molecular “switch”, RhoA GTP can be catalyzed by guanine nucleotide exchange factors (GEFs), which phosphorylate the GDP in GTP10. Y-27632 is a specific Rho-kinase inhibitor, which showed excellent selectivity against RhoA-associated kinases (ROK/ROCK)16, 17.
Gene expression and proteomic analysis of normal hatched, dormant and reactivated blastocysts have indicated differentially expressed RhoA. In fact, RhoA showed higher expression in cryo-dormant blastocysts compared to normal hatched18. Proteomic analysis of dormant and reactivated blastocysts revealed higher levels of RhoA protein in active blastocysts than in the dormant ones19.
Together, such evidences suggest that RhoA gene could provide novel insights into the study of the anti-freezing potentiality of dormant blastocysts in mice. Furthermore, the connections between the role of RhoA and cryopreservation of mammalian blastocysts have never been elucidated. Therefore, in the present study, we have applied both molecular and cellular approaches to contribute to a more detailed knowledge on the role of RhoA in cryopreservation.
Results
RhoA Is Overexpressed in Dormant Blastocysts Compared to Normal Ones
Based on previous findings18, we investigated the expression of RhoA in dormant blastocysts by Real Time-PCR and Western blot. The expression of RhoA mRNA after freezing-thawing step showed a dramatic up-regulation in cryo-dormant embryos compared to cryo-normal and dormant blastocysts (Fig. 1A). Conversely, Western blot results showed upregulation of total RhoA protein in dormant embryos, compared with in normal and cryo-dormant embryos (Fig. 1B).
RhoA gene expression and Western blot analysis (cropped blots) in dormant and normally hatched embryos. (A) RhoA transcription was analysed in various blastocyst groups. Concentrations of RhoA mRNA were normalised to those of GAPDH. Values expressed as means ± SEM, *p < 0.05. (B and C) Western blot profiles of total RhoA normalized with β-actin. Values expressed as mean ± SEM, *p < 0.05.
RhoA GTPases Are Up-Regulated in Dormant Blastocysts
Since phosphorylation is one of the main post-translational protein modifications, we investigated phosphorylated RhoA levels. RhoA and phosphorylation of RhoA (RhoA GTPases) were analysed in embryos using confocal microscopy. The results revealed that the RhoA protein was present only in trophectoderm cells (TE) (Fig. 2A), whereas phosphorylated RhoA (RhoA GTPases) levels were higher in dormant and cryo-dormant blastocysts than in normal and cryo-normal blastocysts (Fig. 2B). In addition, both the total and GTP-bounded (active) RhoA GTPases were up-regulated in TE cells of dormant embryos. Furthermore, differences among RhoA GTPases (Fig. 2B) were remarkable compared with total RhoA levels in embryos (Fig. 2A).
Localisation of total RhoA and phospho-RhoA in mouse embryos (10 × 20 magnification). (A) Images show RhoA antigen labelled in green, propidium iodide labeled nuclei in red, and the merged images. (B) images showing phospho-RhoA antigen labelled in green, Hoechst 33342 labelled nuclei in blue, and the merged images.
RhoA Down-Regulation by Blocking the Rho/ROCK Pathway Decreased Embryos Survival Rates
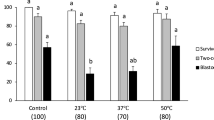
Since RhoA expression analysis showed over-expression in dormant blastocysts, we cultured dormant embryos in-vitro with the specific inhibitor Y-27632, to supress the RhoA-associated kinase (Rho/ROCK) pathway and assess effects on their survival rates after freezing-thawing. The results are reported in Table 1. The supplementation of the medium with Y-27632 significantly decreased the survival rate of both normal and dormant blastocysts (p < 0.01). No significant differences were observed between two control groups for 0 h and 4 h of in-vitro culture.
Recombinant RhoA Protein Improved Survival in Cryopreserved Normal Blastocysts, but Did not Prevent Damage Caused by Y-27632 in Embryos
The survival rate after freezing-thawing of blastocysts co-cultured with recombinant RhoA protein was significantly higher (p < 0.05) than that of control hatched blastocysts (Table 1). In particular, we found that the recombinant RhoA significantly improved the survival rate of normal blastocysts, but did not affect dormant blastocysts. Conversely, the survival rate of blastocysts was decreased when the recombinant protein was used in combination with the inhibitor, showing the dramatic effects of Y-27632 on both normal and dormant embryos (p < 0.01).
Discussions
In our previous study, Gu et al.3 found that dormant blastocysts had a significantly higher survival rate after freezing-thawing than hatched embryos. In a further analysis, Zhang et al.18 also showed that the total RhoA levels in dormant blastocysts were significantly higher than in normally hatched embryos18. Therefore, we further investigated the functional role of RhoA in embryo cryopreservation, using various biological approaches, including different medium formulations for in-vitro culture, Real Time PCR, Western blot and confocal microscopy.
The two cryo-normal groups showed down-regulation compared to the normal groups both in Real-Time PCR and Western blot analysis. It is known that cryopreservation affects transcripts stability making some of them prone to degradation. For instance, in human sperm the cryopreservation affects mRNA-protein interaction making mRNA molecules more susceptible to degradation20. In addition, cryopreservation was reported to produce a decrease in most of gene transcripts and the up-regulation of heat shock protein. This effect was caused by freezing/thawing rather than exposure to cryoprotectants21. This is consistent with our results.
In addition, transcript expression and Western blot analysis showed that RhoA is over-expressed in cryo-dormant group compared to dormant group. We proposed that the up-regulation of RhoA in cryo-dormant blastocysts after thawing contributed to a higher survival rate compared to the normal ones. We expected a consistent trend in the dormant groups, however the results of the Western blot analysis showed an inverse trend. These results evidenced that RhoA expression and translation in blastocysts were inversely correlated. Greenbaum et al.22 have extensively reported the reason for a poor correlation between the level of mRNA and protein. First, there are many complicated and varied post-transcriptional mechanisms involved in turning RhoA mRNA into protein, including phosphorylation (e.g AMPk); second, RhoA protein may differ substantially in their in vivo half-life; third, there is a significant amount of error and noise in both protein and mRNA experiments that limit our ability to get a clear picture. In addition, it should be taken into account that sensitivity of Western blot analysis is affected by the specificity of antibody reactions. In our study, although the reaction was optimised for antibody concentration and incubation time, the anti-RhoA antibody still produced strong background staining, compared with the β-actin control. This may explain the different results obtained with Western blot and Real-time analyses. Nevertheless, RhoA gene expression in dormant blastocysts was higher than normal ones.
RhoA is widely recognised to play important roles in regulating the cytoskeleton and cell division, and it has also been identified as a key mediator of membrane ruffling and lamellae formation23. Therefore, we applied immune-histochemical staining to evaluate the distribution of RhoA protein on different types of blastocysts. Confocal microscopy revealed the exclusive presence of RhoA signal in the embryonic TE cells. In addition, a further experiment demonstrated differences in levels of active phosphorylated RhoA (RhoA GTPases), showing clearly higher staining in dormant and cryo-dormant blastocysts than in normal and cryo-normal hatched ones. These differences among groups strongly supported our hypothesis that the mechanism of anti-freezing in embryos is associated with the expression of RhoA gene or its post-translational regulation (phosphorylation/desphosphorylation) of RhoA. Therefore, investigating RhoA GTPases provided novel insights into the mechanism of anti-freezing in dormant mouse blastocysts.
Recently, Zhang et al.24 showed that, in porcine oocyte meiosis, disruption of RhoA activity and the knock-down of RhoA gene expression caused failure of polar body emission. In mice, the small GTPases RhoA might be a potential upstream regulator of formin-like protein 1 (FMNL1), which affects spindle organisation and action in oocytes polar body extrusion25. Therefore, the RhoA is crucial for embryonic morphogenesis. In our previous study, using transmission electron microscopy, we observed the ultra-structure of dormant blastocyst TE cells3. We proposed that the role of RhoA was connected to the noncanonical Wnt pathway, a signalling process independent from β-catenin regulation26. Down-regulation of RhoA GTPases led to cytoskeletal reorganisation and disassembly of adherens junctions, which destabilize epithelial TE cells to blastocyst-uterine attachment27,28,29. Briefly, the Wnt-RhoA signalling pathway ensures blastocyst competency to implantation and the small GTPases of Rho family are potential mediators of the Wnt pathway. Rho acts downstream, with Rho-associated kinase (ROCK) acting as one effector30. Despite the existence of indistinct signalling networks, the Rho family GTPases and their effectors remain the most obvious link between noncanonical Wnt signalling and the cytoskeleton.
To verify the role of RhoA kinase (post-translational regulation) on blastocyst survival rates after freezing–thawing, three different in vitro culture groups were examined. No blastocysts survived when Y-27632 was added alone, and survival rates were significantly decreased in the presence of a combination of recombinant RhoA and Y-27632, in both normal and dormant blastocysts (p < 0.01). These results suggest that the inhibition of the RhoA/ROCK pathway induced by Y-27632 in mouse blastocysts was non-reversible. Therefore, the specific RhoA inhibitor restrained the survival rate after cryopreservation by blocking the Rho/ROCK signalling pathway.
Supplementation of the medium with recombinant RhoA protein significantly increased the survival rate of normal blastocysts, compared with the controls (p < 0.05), but did not affect dormant blastocysts. However, dormant blastocysts had higher survival rates than the normal blastocysts, with and without recombinant RhoA treatment. Therefore, phosphorylation of recombinant RhoA could only improve the anti-freezing characteristics of normal murine hatched embryos, mediated by the Rho/ROCK signalling pathway, whereas it seems to have not the same effect on dormant embryos. It is likely that the dormant embryos already have a high level of phosphorylated RhoA, so that their own anti-freezing potentiality is not improved by adding further recombinant protein.
RhoA plays a pivotal role in G1 cell cycle progression, primarily through regulation of expression of cyclin D1 and cyclin-dependent kinase inhibitors (p21 and p27). These regulation pathways activate protein kinases, which subsequently modulate transcription factor activity. Entry and maintenance of diapause in dormant blastocysts result from expression of cell cycle inhibitors of the p21 protein family, which interfere with cyclin E-cdk2 complex formation necessary for progression through G1. Therefore, it is possible that basal RhoA levels in dormant blastocysts can activate p21 expression and that cell cycle blockage is already responsible for increasing cryopreservation in the dormant blastocysts. Unlike the normal blastocysts, the dormant ones do not undergo RhoA-induced cell cycle blockage and p21 expression31. Consequently, we suggested two possible pathways to explain why murine dormant blastocysts have higher survival rates than normal ones. In addition, the Rho/ROCK pathway improved the anti-freezing capacity of mouse blastocysts (Fig. 3).
Embryonic diapause occurs because of suppression of cell proliferation at the blastocysts stage. During regulation of the cell cycle in diapause, different proximal signals and cellular factors coordinate with uterine factors to arrest the developmental stage. During this phase, traces of ovarian oestrogens can induce mitotic restart of embryos1. Additionally, the metabolic activities of dormant blastocysts are different from those of the activated blastocysts19. Dormant embryos before freezing are characterized by harsh living conditions and their energy metabolism is reduced to a “basal level”, which allows them to have better anti-freezing properties than normally hatched blastocysts3. However, it remains unknown which event triggers the embryonic anti-freezing mechanisms during cryopreservation. Combining molecular, physiological and pharmacological approaches, we showed that the Rho/ROCK signalling pathway plays key roles in murine blastocysts cryopreservation. Similarly, we demonstrated failure of the blastocysts survival when the Rho/ROCK signalling pathway was blocked by Y-27632. This is consistent with the finding that RhoA down-regulation destabilised the edges of TE cells and weakened the cytoskeleton in embryos29. Our results suggest that up-regulation of RhoA and RhoA GTPases in dormant embryos could reinforce the cytoskeleton and, therefore, improve the anti-freezing potentiality of murine hatched and dormant blastocysts.
These findings increased knowledge of mammalian cryopreservation mechanisms, suggesting that the Rho/ROCK signalling pathway can be one of the main key points to determine the fate of the embryos during the cryopreservation. Furthermore, the supplementation with recombinant RhoA protein could increase the survival of blastocysts, indicating that it is a protective anti-freezing additive. However, this aspect has to be further clarified, whereas RhoA phosphorylation, mediated by the Rho/ROCK pathway, might improve the anti-freezing potential of murine hatched and delayed blastocysts.
Materials and Methods
Ethical approval
The Ethics Committee of Beijing University of Agriculture approved the study (Permit Number 2012-0611) and all the methods were carried out in accordance with the approved guidelines.
Mice were bought from Beijing Vital River Laboratory Animal Technology Co., Ltd. (SCXK Beijing 2012-0001) and housed in the Institutional Animal Care Facility of Beijing University of Agriculture (SYXK Beijing 2010-0003) according to institutional guidelines for laboratory. Procedures were conducted in accordance with the ethical standards of Ministry of Science and Technology of China applied by the Institutional Animal Care Committee of the Beijing University of Agriculture. No endangered or protected species were involved in this study.
Animal Models and Blastocysts
A total of 200 female ICR mice, 6–8 week old, were used for the present study. Superovulation was induced by intraperitoneal injection of 10 IU pregnant mare serum gonadotropin (PMSG) and human chorionic gonadotropin (hCG), given 46–48 h before mating with 12 week old ICR males. Mating was confirmed by the presence of a copulation plug in the morning and, then, 10 IU of anti-pregnant mare’s serum gonadotropin (A-PMSG) was administered to the females by injection32. Mice were bilaterally ovariectomised in the morning (08.00–09.00 h) of day 4, and then they were treated daily with 0.2 g progesterone, prepared in 10 ml sesame oil until day 7 to induce delayed implantation. A total of 800 dormant blastocysts were collected on day 8. In addition, 800 normally hatched blastocysts were collected from control intact mice on day 5.
Freezing-thawing of Embryos
Harvested mouse blastocysts were washed twice with PBS (Sigma-Aldrich, St. louis, MO, USA) and then washed twice in frozen fluid (ICPbio Reproduction; 101129, Auckland, New Zeland) to perform the freezing step. Briefly, the mouse embryos were aspirated into a 0.25 ml plastic straw. The cooling rate of the freezing apparatus was set up to −1 °C/min until samples reached a temperature of −5 °C. Within 10 min, using tweezers, the straws containing the embryos were cooled down, showing an ice layer on the top within 3–5 s. Then, frozen tubes were stored into liquid nitrogen until the cooling rate was changed to −0.3 °C/min until the temperature reached −35 °C and then stored in liquid nitrogen.
To thaw samples, the frozen tubes were removed from liquid nitrogen, gently shacked at room temperature for 5–10 s, and moved into a 35 °C water bath for 10 s. Afterwards, the blastocysts were quickly transferred to a glass dish containing 1 M sucrose solution.
Quantitative Real Time PCR Analysis (Q-PCR)
Comparative RhoA gene expression was accomplished in 4 groups of embryos, normal hatched and dormant embryos, each before and after the cryopreservation treatment. A total of 100 blastocysts in each group were used to extract total RNA. Reverse transcription reactions were performed using oligo dT18. Real-time PCR was performed using the CFX96 Touch Real-Time PCR Detection SYBR Green Supermix (BioRad, Hercules, CA, USA). The following RhoA gene specific primers were used for real-time amplification: forward 5′-ACT CGG AGT CCT CGC CTT GA-3′ and reverse 5′-AAG CTC CAT CAC CAA CAA TCA C-3′ (GenBank accession number NM_ 016802.5). GAPDH was chosen as the housekeeping gene (GenBank accession number NM_ 008084.3) and amplified with the following primers: forward 5′-TGG CAA AGT GGA GAT TGT TGC C-3′ and reverse 5′-AAG ATG GTA ATA AAC TTC CCG-3′. Samples were run in triplicate, and the relative gene expression was calculated using the comparative threshold cycle (Ct) and normalised to expression of GAPDH (ΔCt). Results are expressed as fold change relative to the mean.
Western Blot Analysis of Blastocysts
A total of 200 blastocysts were collected for each treatment condition. Samples were boiled in 5X loading buffer for 10 min and rapidly transferred to ice. Protein samples were electrophoresed using 10% SDS-polyacrylamide gels and bands were transferred to a PVDF membrane (Millipore, MA, USA). The membranes were blocked for 2 h in PBS with 0.1% Tween-20 (PBST) containing 5% of skim milk and then were incubated overnight at 4 °C with primary antibodies (1:1000 dilution). A mouse monoclonal β-actin antibody (Sigma-Aldrich) at 1:2000 dilution was used to control for sample loading. After washing 3 times with PBST, the membranes were incubated at 37 °C for 1 h with a donkey anti-rabbit immunoglobulin (IgG) secondary antibody (1:10000 dilution; Zhongshan Golden Bridge Biotechnology Co., Ltd, China). Protein bands were visualised using the ECL Plus Western Blotting Detection System Tanon-5500 (BioRad). To quantify western blot results, band intensity values were determined using Image J software (National Institute of Health; Bethesda, MD, USA).
Immunofluorescence Staining and Confocal Microscopy
For observing the distribution of total RhoA and the active RhoA-GTP, immunofluorescence staining of blastocysts was performed as described by Wang et al.33 using an anti-RhoA antibody (ab86279, Abcam) and an anti-active RhoA antibody (26904, New East Biosciences, King of Prussia, PA, USA). Slides were observed by confocal scanning laser microscope (LSM 710 model; Zeiss, Jena, Germany) and images analysed with Zeiss LSM image browsing software.
Evaluation of Effects of Y-27632 and Recombinant RhoA on Embryonic Viability after Cryopreservation
To determine the effects of Y-27632 on blastocysts, the normal and dormant embryos were cultured in Whitten’s solution supplemented with various concentrations of Y-27632 (0, 1, 10, 20, 100 μM; Sigma-Aldrich) for 4 h. A Y-27632 concentration of 20 μM was determined to be optimal. Recombined RhoA protein (Sino Biological Inc., Beijing, China) was diluted with the culture medium for optimal storage (100 μg/μl). Final concentration (1, 10, 20, 50 μg/μl) was tested in culture medium with embryos. A concentration of 10 μg/μl was then used to culture blastocysts for 4 h. The embryos were cultured in 50 μl drops of fresh pre-equilibrated culture medium at 37 °C in 5% CO2. Three replicate experiments were conducted, treating and evaluating a total of 360 embryos.
After freezing-thawing, the embryos were washed in Whitten’s solution and cultured in the same medium, covered with mineral oil at 37 °C in 5% CO2 and saturated humidity. Embryos showing re-expansion in post-thawing/warming cultures were considered to be alive. The percentages of embryos showing re-expansion were determined at 4 h intervals during 24 h of additional culture after thawing/warming. In various treatment groups, the percentage of re-expanded blastocysts, determined at each time point, were used to calculate the survival rates4.
Statistical Analysis
Data are presented as means ± SEM. Statistical comparisons of data were performed using analysis of variance (ANOVA), followed by Student-Newman-Keuls test, calculated with SPSS software (IBM, New York, USA). P-values lower than 0.05 were considered significant.
References
Lopes, F. L., Desmarais, J. A. & Murphy, B. D. Embryonic diapause and its regulation. Reproduction 128, 669–678 (2004).
Dey, S. et al. Molecular cues to implantation. Endocrine reviews 25, 341–373 (2004).
Gu, M. et al. Ultrastructural observation of dormant mouse embryos cultured in vitro after freezing-thawing. Acta Laboratorium Animalis Scientia Sinica 22(53–56), 61 (2014).
Inaba, Y. et al. Cryopreservation method affects DNA fragmentation in trophectoderm and the speed of re-expansion in bovine blastocysts. Cryobiology 72, 86–92 (2016).
Morató, R. et al. Effects of pre‐treating in vitro‐matured bovine oocytes with the cytoskeleton stabilizing agent taxol prior to vitrification. Molecular reproduction and development 75, 191–201 (2008).
Wang, C. et al. Stability of the cytoskeleton of matured buffalo oocytes pretreated with cytochalasin B prior to vitrification. Cryobiology 72, 274–282 (2016).
Ahn, H. J. et al. Characteristics of the cell membrane fluidity, actin fibers, and mitochondrial dysfunctions of frozen‐thawed two‐cell mouse embryos. Molecular reproduction and development 61, 466–476 (2002).
Mo, X. et al. Effect of meiotic status, cumulus cells and cytoskeleton stabilizer on the developmental competence of ovine oocytes following vitrification. Small Ruminant Research 117, 151–157 (2014).
Machacek, M. et al. Coordination of Rho GTPase activities during cell protrusion. Nature 461, 99–103 (2009).
Jordan, S. N. & Canman, J. C. Rho GTPases in animal cell cytokinesis: an occupation by the one percent. Cytoskeleton 69, 919–930 (2012).
Kishi, K., Sasaki, T., Kuroda, S., Itoh, T. & Takai, Y. Regulation of cytoplasmic division of Xenopus embryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI). The Journal of Cell Biology 120, 1187–1195 (1993).
Prokopenko, S. N. et al. A putative exchange factor for Rho1 GTPase is required for initiation of cytokinesis in Drosophila. Genes & development 13, 2301–2314 (1999).
Crawford, J. M., Harden, N., Leung, T., Lim, L. & Kiehart, D. P. Cellularization inDrosophila melanogasterIs Disrupted by the Inhibition of Rho Activity and the Activation of Cdc42 Function. Developmental biology 204, 151–164 (1998).
Lai, S. L., Chang, C. N., Wang, P. J. & Lee, S. J. Rho mediates cytokinesis and epiboly via ROCK in zebrafish. Molecular reproduction and development 71, 186–196 (2005).
Hirai, A. et al. Geranylgeranylated rho small GTPase (s) are essential for the degradation of p27Kip1 and facilitate the progression from G1 to S phase in growth-stimulated rat FRTL-5 cells. Journal of Biological Chemistry 272, 13–16 (1997).
Uehata, M. et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389, 990–994 (1997).
Ishizaki, T. et al. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Molecular pharmacology 57, 976–983 (2000).
Zhang, S. et al. Gene expression profile analysis of dormant mouse embryos preserved by controlled slow freezing. Acta Laboratorium Animalis Scientia Sinica 20, 15–19 (2012).
Fu, Z. et al. Integral proteomic analysis of blastocysts reveals key molecular machinery governing embryonic diapause and reactivation for implantation in mice. Biology of reproduction 90, 52 (2014).
Flores, E. et al. Freezing-thawing induces alterations in histone H1-DNA binding and the breaking of protein-DNA disulfide bonds in boar sperm. Theriogenology 76, 1450–1464 (2011).
Riesco, M. F. & Robles, V. Cryopreservation causes genetic and epigenetic changes in zebrafish genital ridges. PloS one 8, e67614 (2013).
Greenbaum, D., Colangelo, C., Williams, K. & Gerstein, M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome biology 4, 117 (2003).
Wheeler, A. P. & Ridley, A. J. Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Experimental cell research 301, 43–49 (2004).
Zhang, Y. et al. Small GTPase RhoA regulates cytoskeleton dynamics during porcine oocyte maturation and early embryo development. Cell cycle 13, 3390–3403 (2014).
Wang, F. et al. RhoA-mediated FMNL1 regulates GM130 for actin assembly and phosphorylates MAPK for spindle formation in mouse oocyte meiosis. Cell Cycle 14, 2835–2843 (2015).
Veeman, M. T., Axelrod, J. D. & Moon, R. T. A second canon: functions and mechanisms of β-catenin-independent Wnt signaling. Developmental cell 5, 367–377 (2003).
Braga, V. M., Machesky, L. M., Hall, A. & Hotchin, N. A. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell–cell contacts. The Journal of cell biology 137, 1421–1431 (1997).
Sahai, E. & Marshall, C. J. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nature cell biology 4, 408–415 (2002).
Xie, H. et al. Inactivation of nuclear Wnt-β-catenin signaling limits blastocyst competency for implantation. Development 135, 717–727 (2008).
Winter, C. G. et al. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell 105, 81–91 (2001).
Bokoch, G. M. Biology of the p21-activated kinases. Annual review of biochemistry 72, 743–781 (2003).
Lu, T. et al. A New Method to Obtain Murine Dormant Embryos by Superovulation Combined with Anti-PMSG Serum. Acta Laboratorium Animalis Scientia Sinica 212–214 (2007).
Wang, H. et al. Differential G protein-coupled cannabinoid receptor signaling by anandamide directs blastocyst activation for implantation. Proceedings of the National Academy of Sciences 100, 14914–14919 (2003).
Acknowledgements
We are grateful to Chundong Zhai for assistance in embryo collection and Fu Zheng for assistance with confocal microscopy. This study was supported by the National Fund for Natural Sciences of China (Grant no. 31272526) and by the Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges, under the Beijing municipality (Grant no. PXM2013_014207_000067).
Author information
Authors and Affiliations
Contributions
Meichao Gu conceived, designed and performed most of the experiments and wrote the paper. Hemin Ni analysed and reviewed the results. Xihui Sheng conducted experiments investigating signalling pathways. Alfredo Pauciullo critically revised the article for intellectual content. Yunhai Liu managed the experimental procedures. Yong Guo conceived the idea for the project and reviewed the paper. All authors approved the final version of this manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gu, M., Ni, H., Sheng, X. et al. RhoA phosphorylation mediated by Rho/RhoA-associated kinase pathway improves the anti-freezing potentiality of murine hatched and diapaused blastocysts. Sci Rep 7, 6705 (2017). https://doi.org/10.1038/s41598-017-07066-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-07066-2
This article is cited by
-
Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome
Nature Medicine (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.