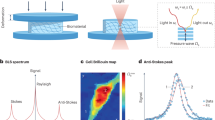
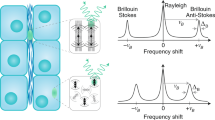
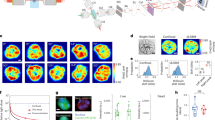
Abstract
Several techniques have been developed over the past few decades to assess the mechanical properties of biological samples, which has fueled a rapid growth in the fields of biophysics, bioengineering, and mechanobiology. In this context, Brillouin optical spectroscopy has long been known as an intriguing modality for noncontact material characterization. However, limited by speed and sample damage, it had not translated into a viable imaging modality for biomedically relevant materials. Recently, based on a novel spectroscopy strategy that substantially improves the speed of Brillouin measurement, confocal Brillouin microscopy has emerged as a unique complementary tool to traditional methods as it allows noncontact, nonperturbative, label-free measurements of material mechanical properties. The feasibility and potential of this innovative technique at both the cell and tissue level have been extensively demonstrated over the past decade. As Brillouin technology is rapidly recognized, a standard approach for building and operating Brillouin microscopes is required to facilitate the widespread adoption of this technology. In this protocol, we aim to establish a robust approach for instrumentation, and data acquisition and analysis. By carefully following this protocol, we expect that a Brillouin instrument can be built in 5–9 days by a person with basic optics knowledge and alignment experience; the data acquisition as well as postprocessing can be accomplished within 2–8 h.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its Supplementary Information files. The raw Brillouin spectra of Figs. 8 and 9 are available via Figshare (https://figshare.com/articles/dataset/raw_data_to_Fig_8_9/13135760). Source data are provided with this paper.
Code availability
The MATLAB code to analyze images as well as representative raw data are provided as Supplementary Data 1.
References
Vogel, V. & Sheetz, M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 7, 265–275 (2006).
Discher, D. E., Janmey, P. & Wang, Y.-L. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005).
Wozniak, M. A. & Chen, C. S. Mechanotransduction in development: a growing role for contractility. Nat. Rev. Mol. Cell Biol. 10, 34–43 (2009).
Miller, C. J. & Davidson, L. A. The interplay between cell signalling and mechanics in developmental processes. Nat. Rev. Genet. 14, 733–744 (2013).
Gilkes, D. M., Semenza, G. L. & Wirtz, D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat. Rev. Cancer 14, 430–439 (2014).
Orr, A. W., Helmke, B. P., Blackman, B. R. & Schwartz, M. A. Mechanisms of mechanotransduction. Dev. Cell 10, 11–20 (2006).
Polacheck, W. J. & Chen, C. S. Measuring cell-generated forces: a guide to the available tools. Nat. Methods 13, 415–423 (2016).
Bao, G. & Suresh, S. Cell and molecular mechanics of biological materials. Nat. Mater. 2, 715–725 (2003).
Campas, O. A toolbox to explore the mechanics of living embryonic tissues. Semin. Cell Dev. Biol. 55, 119–130 (2016).
Heinisch, J. J., Dupres, V., Alsteens, D. & Dufrêne, Y. F. Measurement of the mechanical behavior of yeast membrane sensors using single-molecule atomic force microscopy. Nat. Protoc. 5, 670 (2010).
Stewart, M. P., Toyoda, Y., Hyman, A. A. & Müller, D. J. Tracking mechanics and volume of globular cells with atomic force microscopy using a constant-height clamp. Nat. Protoc. 7, 143 (2012).
Benaglia, S., Gisbert, V. G., Perrino, A. P., Amo, C. A. & Garcia, R. Fast and high-resolution mapping of elastic properties of biomolecules and polymers with bimodal AFM. Nat. Protoc. 13, 2890–2907 (2018).
Efremov, Y. M., Cartagena-Rivera, A. X., Athamneh, A. I., Suter, D. M. & Raman, A. Mapping heterogeneity of cellular mechanics by multi-harmonic atomic force microscopy. Nat. Protoc. 13, 2200–2216 (2018).
Krieg, M. et al. Atomic force microscopy-based mechanobiology. Nat. Rev. Phys. 1, 41–57 (2019).
Trier, S. M. & Davidson, L. A. Quantitative microscopy and imaging tools for the mechanical analysis of morphogenesis. Curr. Opin. Genet. Dev. 21, 664–670 (2011).
Chevalier, N. R., Gazguez, E., Dufour, S. & Fleury, V. Measuring the micromechanical properties of embryonic tissues. Methods 94, 120–128 (2016).
Evans, E. & Yeung, A. Apparent viscosity and cortical tension of blood granulocytes determined by micropipet aspiration. Biophys. J. 56, 151–160 (1989).
Hochmuth, R. M. Micropipette aspiration of living cells. J. Biomech. 33, 15–22 (2000).
Desprat, N., Guiroy, A. & Asnacios, A. Microplates-based rheometer for a single living cell. Rev. Sci. Instrum. 77, 055111 (2006).
Caille, N., Tardy, Y. & Meister, J.-J. Assessment of strain field in endothelial cells subjected to uniaxial deformation of their substrate. Ann. Biomed. Eng. 26, 409–416 (1998).
Svoboda, K. & Block, S. M. Biological applications of optical forces. Annu. Rev. Biophys. Biomol. Struct. 23, 247–285 (1994).
Lee, W. M., Reece, P. J., Marchington, R. F., Metzger, N. K. & Dholakia, K. Construction and calibration of an optical trap on a fluorescence optical microscope. Nat. Protoc. 2, 3226 (2007).
Wang, N., Butler, J. P. & Ingber, D. E. Mechanotransduction across the cell surface and through the cytoskeleton. Science 260, 1124–1127 (1993).
Zhang, Y. et al. Interfacing 3D magnetic twisting cytometry with confocal fluorescence microscopy to image force responses in living cells. Nat. Protoc. 12, 1437 (2017).
Bausch, A. R., Möller, W. & Sackmann, E. Measurement of local viscoelasticity and forces in living cells by magnetic tweezers. Biophys. J. 76, 573–579 (1999).
Mason, T., Ganesan, K., Van Zanten, J., Wirtz, D. & Kuo, S. C. Particle tracking microrheology of complex fluids. Phys. Rev. Lett. 79, 3282 (1997).
Serwane, F. et al. In vivo quantification of spatially varying mechanical properties in developing tissues. Nat. Methods 14, 181–186 (2017).
Guck, J. et al. The optical stretcher: a novel laser tool to micromanipulate cells. Biophys. J. 81, 767–784 (2001).
Wang, S. & Larin, K. V. Optical coherence elastography for tissue characterization: a review. J. Biophotonics 8, 279–302 (2015).
Kennedy, B. F., Wijesinghe, P. & Sampson, D. D. The emergence of optical elastography in biomedicine. Nat. Photonics 11, 215–221 (2017).
Gossett, D. R. et al. Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc. Natl Acad. Sci. USA 109, 7630–7635 (2012).
Otto, O. et al. Real-time deformability cytometry: on-the-fly cell mechanical phenotyping. Nat. Methods 12, 199–202 (2015).
Hartono, D. et al. On-chip measurements of cell compressibility via acoustic radiation. Lab Chip 11, 4072–4080 (2011).
Kang, J. H. et al. Noninvasive monitoring of single-cell mechanics by acoustic scattering. Nat. Methods 16, 263–269 (2019).
Dil, J. G. Brillouin scattering in condensed matter. Rep. Prog. Phys. 45, 285–334 (1982).
Scarcelli, G. & Yun, S. H. Confocal Brillouin microscopy for three-dimensional mechanical imaging. Nat. Photonics 2, 39–43 (2008).
Prevedel, R., Diz-Muñoz, A., Ruocco, G. & Antonacci, G. Brillouin microscopy: an emerging tool for mechanobiology. Nat. Methods 16, 969–977 (2019).
Palombo, F. & Fioretto, D. Brillouin light scattering: applications in biomedical sciences. Chem. Rev. 119, 7833–7847 (2019).
Scarcelli, G. et al. Noncontact three-dimensional mapping of intracellular hydro-mechanical properties by Brillouin microscopy. Nat. Methods 12, 1132–1134 (2015).
Scarcelli, G., Kim, P. & Yun, S. H. In vivo measurement of age-related stiffening in the crystalline lens by Brillouin optical microscopy. Biophys. J. 101, 1539–1545 (2011).
Scarcelli, G., Pineda, R. & Yun, S. H. Brillouin optical microscopy for corneal biomechanics. Investig. Ophthalmol. Vis. Sci. 53, 185–190 (2012).
Scarcelli, G., Besner, S., Pineda, R. & Yun, S. H. Biomechanical characterization of keratoconus corneas ex vivo with Brillouin microscopy. Investig. Ophthalmol. Vis. Sci. 55, 4490–4495 (2014).
Scarcelli, G., Besner, S., Pineda, R., Kalout, P. & Yun, S. H. In vivo biomechanical mapping of normal and keratoconus corneas. JAMA Ophthalmol. 133, 480–482 (2015).
Elsayad, K. et al. Mapping the subcellular mechanical properties of live cells in tissues with fluorescence emission-Brillouin imaging. Sci. Signal 9, rs5 (2016).
Schlüßler, R. et al. Mechanical mapping of spinal cord growth and repair in living zebrafish larvae by brillouin imaging. Biophys. J. 115, 911–923 (2018).
Zhang, J. et al. Tissue biomechanics during cranial neural tube closure measured by Brillouin microscopy and optical coherence tomography. Birth Defects Res. 111, 991–998 (2018).
Antonacci, G., de Turris, V., Rosa, A. & Ruocco, G. Background-deflection Brillouin microscopy reveals altered biomechanics of intracellular stress granules by ALS protein FUS. Commun. Biol. 1, 1–8 (2018).
Gouveia, R. M. et al. Assessment of corneal substrate biomechanics and its effect on epithelial stem cell maintenance and differentiation. Nat. Commun. 10, 1496 (2019).
Margueritat, J. et al. High-frequency mechanical properties of tumors measured by Brillouin light scattering. Phys. Rev. Lett. 122, 018101 (2019).
Conrad, C., Gray, K. M., Stroka, K. M., Rizvi, I. & Scarcelli, G. Mechanical characterization of 3D ovarian cancer nodules using Brillouin confocal microscopy. Cell. Mol. Bioeng. 12, 215–226 (2019).
Zhang, J. et al. Nuclear mechanics within intact cells is regulated by cytoskeletal network and internal nanostructures. Small 16, 1907688 (2020).
Lindsay, S., Anderson, M. & Sandercock, J. Construction and alignment of a high performance multipass vernier tandem Fabry–Perot interferometer. Rev. Sci. Instrum. 52, 1478–1486 (1981).
Hickman, G. D. et al. Aircraft laser sensing of sound velocity in water: Brillouin scattering. Remote Sens. Environ. 36, 165–178 (1991).
Harley, R., James, D., Miller, A. & White, J. Phonons and the elastic moduli of collagen and muscle. Nature 267, 285–287 (1977).
Cusack, S. & Miller, A. Determination of the elastic constants of collagen by Brillouin light scattering. J. Mol. Biol. 135, 39–51 (1979).
Randall, J. T. & Vaughan, J. M. Brillouin scattering in systems of biological significance. Philos. Trans. R. Soc. Lond. A 293, 341–348 (1979).
Vaughan, J. & Randall, J. Brillouin scattering, density and elastic properties of the lens and cornea of the eye. Nature 284, 489–491 (1980).
Randall, J. & Vaughan, J. The measurement and interpretation of Brillouin scattering in the lens of the eye. Proc. R. Sci. Lond. B 214, 449–470 (1982).
Lee, S. et al. A Brillouin scattering study of the hydration of Li-and Na-DNA films. Biopolymers 26, 1637–1665 (1987).
Lees, S., Tao, N.-J. & Lindsay, S. Studies of compact hard tissues and collagen by means of Brillouin light scattering. Connect. Tissue Res. 24, 187–205 (1990).
Itoh, S.-i, Yamana, T. & Kojima, S. Quick measurement of Brillouin spectra of glass-forming material trimethylene glycol by angular dispersion-type Fabry–Perot interferometer system. Jpn. J. Appl. Phys. 35, 2879 (1996).
Koski, K. & Yarger, J. Brillouin imaging. Appl. Phys. Lett. 87, 061903 (2005).
Shirasaki, M. Large angular dispersion by a virtually imaged phased array and its application to a wavelength demultiplexer. Opt. Lett. 21, 366–368 (1996).
Scarcelli, G. & Yun, S. H. Multistage VIPA etalons for high-extinction parallel Brillouin spectroscopy. Opt. Express 19, 10913–10922 (2011).
Berghaus, K. V., Yun, S. H. & Scarcelli, G. High speed sub-GHz spectrometer for Brillouin scattering analysis. J. Vis. Exp. 106, e53468 (2015).
Antonacci, G., Lepert, G., Paterson, C. & Török, P. Elastic suppression in Brillouin imaging by destructive interference. Appl. Phys. Lett. 107, 061102 (2015).
Meng, Z., Traverso, A. J. & Yakovlev, V. V. Background clean-up in Brillouin microspectroscopy of scattering medium. Opt. Express 22, 5410–5415 (2014).
Fiore, A., Zhang, J., Shao, P., Yun, S. H. & Scarcelli, G. High-extinction virtually imaged phased array-based Brillouin spectroscopy of turbid biological media. Appl. Phys. Lett. 108, 203701 (2016).
Edrei, E., Gather, M. C. & Scarcelli, G. Integration of spectral coronagraphy within VIPA-based spectrometers for high extinction Brillouin imaging. Opt. Express 25, 6895–6903 (2017).
Nikolić, M. & Scarcelli, G. Long-term Brillouin imaging of live cells with reduced absorption-mediated damage at 660nm wavelength. Biomed. Opt. Express 10, 1567–1580 (2019).
Zhang, J., Fiore, A., Yun, S.-H., Kim, H. & Scarcelli, G. Line-scanning Brillouin microscopy for rapid non-invasive mechanical imaging. Sci. Rep. 6, 35398 (2016).
Raghunathan, R. et al. Evaluating biomechanical properties of murine embryos using Brillouin microscopy and optical coherence tomography. J. Biomed. Opt. 22, 086013 (2017).
Roberts C. J. Biomechanics in keratoconus. in Textbook of Keratoconus: New Insights (ed. Barbara, A.) 29–32 (Jaypee Brothers Medical Publishers, 2012).
Yun, S. H. & Chernyak, D. Brillouin microscopy: assessing ocular tissue biomechanics. Curr. Opin. Ophthalmol. 29, 299 (2018).
Webb, J. N., Zhang, H., Roy, A. S., Randleman, J. B. & Scarcelli, G. Detecting mechanical anisotropy of the cornea using Brillouin microscopy. Transl. Vis. Sci. Technol. 9, 26–26 (2020).
Eltony, A. M., Shao, P. & Yun, S.-H. Measuring mechanical anisotropy of the cornea with Brillouin microscopy. Preprint at https://arxiv.org/abs/2003.04344 (2020).
Shao, P. et al. Spatially-resolved Brillouin spectroscopy reveals biomechanical abnormalities in mild to advanced keratoconus in vivo. Sci. Rep. 9, 1–12 (2019).
Reiß, S., Burau, G., Stachs, O., Guthoff, R. & Stolz, H. Spatially resolved Brillouin spectroscopy to determine the rheological properties of the eye lens. Biomed. Opt. Express 2, 2144–2159 (2011).
Besner, S., Scarcelli, G., Pineda, R. & Yun, S.-H. In vivo Brillouin analysis of the aging crystalline lens. Investig. Ophthalmol. Vis. Sci. 57, 5093–5100 (2016).
Weber, I. P., Yun, S. H., Scarcelli, G. & Franze, K. The role of cell body density in ruminant retina mechanics assessed by atomic force and Brillouin microscopy. Phys. Biol. 14, 065006 (2017).
Shawky, J. H. & Davidson, L. A. Tissue mechanics and adhesion during embryo development. Dev. Biol. 401, 152–164 (2015).
Bevilacqua, C., Sánchez-Iranzo, H., Richter, D., Diz-Muñoz, A. & Prevedel, R. Imaging mechanical properties of sub-micron ECM in live zebrafish using Brillouin microscopy. Biomed. Opt. Express 10, 1420–1431 (2019).
Wang, N., Tytell, J. D. & Ingber, D. E. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 10, 75–82 (2009).
Kirby, T. J. & Lammerding, J. Emerging views of the nucleus as a cellular mechanosensor. Nat. Cell Biol. 20, 373–381 (2018).
Meng, Z., Bustamante Lopez, S. C., Meissner, K. E. & Yakovlev, V. V. Subcellular measurements of mechanical and chemical properties using dual Raman–Brillouin microspectroscopy. J. Biophotonics 9, 201–207 (2016).
Antonacci, G. & Braakman, S. Biomechanics of subcellular structures by non-invasive Brillouin microscopy. Sci. Rep. 6, 37217 (2016).
Altartouri, B. et al. Pectin chemistry and cellulose crystallinity govern pavement cell morphogenesis in a multi-step mechanism. Plant Physiol. 181, 127–141 (2019).
Zhang, J., Nou, X. A., Kim, H. & Scarcelli, G. Brillouin flow cytometry for label-free mechanical phenotyping of the nucleus. Lab Chip 17, 663–670 (2017).
Suresh, S. Biomechanics and biophysics of cancer cells. Acta Mater. 55, 3989–4014 (2007).
Guck, J. et al. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys. J. 88, 3689–3698 (2005).
Cross, S. E., Jin, Y.-S., Rao, J. & Gimzewski, J. K. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2, 780 (2007).
Li, Q., Lee, G. Y., Ong, C. N. & Lim, C. T. AFM indentation study of breast cancer cells. Biochem. Biophys. Res. Commun. 374, 609–613 (2008).
Plodinec, M. et al. The nanomechanical signature of breast cancer. Nat. Nanotechnol. 7, 757–765 (2012).
Prabhune, M., Belge, G., Dotzauer, A., Bullerdiek, J. & Radmacher, M. Comparison of mechanical properties of normal and malignant thyroid cells. Micron 43, 1267–1272 (2012).
Wisniewski, E. et al. Dorsoventral polarity directs cell responses to migration track geometries. Sci. Adv. 6, eaba6505 (2020).
Koski, K. J., Akhenblit, P., McKiernan, K. & Yarger, J. L. Non-invasive determination of the complete elastic moduli of spider silks. Nat. Mater. 12, 262–267 (2013).
Mercatelli, R. et al. Morpho-mechanics of human collagen superstructures revealed by all-optical correlative micro-spectroscopies. Commun. Biol. 2, 1–10 (2019).
Palombo, F. et al. Biomechanics of fibrous proteins of the extracellular matrix studied by Brillouin scattering. J. R. Soc. Interface 11, 20140739 (2014).
Speziale, S. et al. Sound velocity and elasticity of tetragonal lysozyme crystals by Brillouin spectroscopy. Biophys. J. 85, 3202–3213 (2003).
Yan, K. et al. Electrical programming of soft matter: using temporally varying electrical inputs to spatially control self assembly. Biomacromolecules 19, 364–373 (2018).
Meng, Z. et al. Assessment of local heterogeneity in mechanical properties of nanostructured hydrogel networks. ACS Nano 11, 7690–7696 (2017).
Bailey, M. et al. Brillouin microspectroscopy data of tissue-mimicking gelatin hydrogels. Data Brief. 29, 105267 (2020).
Antonacci, G. et al. Quantification of plaque stiffness by Brillouin microscopy in experimental thin cap fibroatheroma. J. R. Soc. Interface 12, 20150843 (2015).
Steelman, Z., Meng, Z., Traverso, A. J. & Yakovlev, V. V. Brillouin spectroscopy as a new method of screening for increased CSF total protein during bacterial meningitis. J. Biophotonics 8, 408–414 (2015).
Mattana, S., Caponi, S., Tamagnini, F., Fioretto, D. & Palombo, F. Viscoelasticity of amyloid plaques in transgenic mouse brain studied by Brillouin microspectroscopy and correlative Raman analysis. J. Innov. Opt. Health Sci. 10, 1742001 (2017).
Palombo, F. et al. Hyperspectral analysis applied to micro-Brillouin maps of amyloid-beta plaques in Alzheimer’s disease brains. Analyst 143, 6095–6102 (2018).
Troyanova-Wood, M., Gobbell, C., Meng, Z., Gashev, A. A. & Yakovlev, V. V. Optical assessment of changes in mechanical and chemical properties of adipose tissue in diet‐induced obese rats. J. Biophotonics 10, 1694–1702 (2017).
Troyanova-Wood, M., Meng, Z. & Yakovlev, V. V. Differentiating melanoma and healthy tissues based on elasticity-specific Brillouin microspectroscopy. Biomed. Opt. Express 10, 1774–1781 (2019).
Lainović, T. et al. Micromechanical imaging of dentin with Brillouin microscopy. Acta Biomater. 105, 214–222 (2020).
Stephen, M. A., Krainak, M. A. & Fahey, M. E. Lateral-transfer recirculating etalon spectrometer. Opt. Express 23, 30020–30027 (2015).
Scarponi, F. et al. High-performance versatile setup for simultaneous Brillouin–Raman microspectroscopy. Phys. Rev. X 7, 031015 (2017).
Mattana, S. et al. Non-contact mechanical and chemical analysis of single living cells by microspectroscopic techniques. Light Sci. Appl. 7, 17139–17139 (2018).
Schneider, D. et al. Nonlinear control of high-frequency phonons in spider silk. Nat. Mater. 15, 1079–1083 (2016).
Boyd, R. W. Nonlinear Optics (Academic Press, 2003).
Ballmann, C. W. et al. Stimulated Brillouin scattering microscopic imaging. Sci. Rep. 5, 18139 (2015).
Remer, I. & Bilenca, A. Background-free Brillouin spectroscopy in scattering media at 780 nm via stimulated Brillouin scattering. Opt. Lett. 41, 926–929 (2016).
Remer, I. & Bilenca, A. High-speed stimulated Brillouin scattering spectroscopy at 780 nm. Appl. Photonics 1, 061301 (2016).
Remer, I., Shemsesh, N., Ben-Zvi, A. & Bilenca, A. High sensitivity and specificity biomechanical imaging by stimulated Brillouin scattering microscopy. Nat. Methods 17, 913–916 (2020).
Nelson, K. A., Miller, R. D., Lutz, D. & Fayer, M. Optical generation of tunable ultrasonic waves. J. Appl. Phys. 53, 1144–1149 (1982).
Ballmann, C. W., Meng, Z., Traverso, A. J., Scully, M. O. & Yakovlev, V. V. Impulsive Brillouin microscopy. Optica 4, 124–128 (2017).
Krug, B., Koukourakis, N. & Czarske, J. W. Impulsive stimulated Brillouin microscopy for non-contact, fast mechanical investigations of hydrogels. Opt. Express 27, 26910–26923 (2019).
Guo, M. et al. Cell volume change through water efflux impacts cell stiffness and stem cell fate. Proc. Natl Acad. Sci. USA 114, E8618–E8627 (2017).
Wu, P.-J. et al. Water content, not stiffness, dominates Brillouin spectroscopy measurements in hydrated materials. Nat. Methods 15, 561 (2018).
Scarcelli, G. & Yun, S. H. Reply to ‘Water content, not stiffness, dominates Brillouin spectroscopy measurements in hydrated materials’. Nat. Methods 15, 562 (2018).
Xiao, S., Weiner, A. M. & Lin, C. A dispersion law for virtually imaged phased-array spectral dispersers based on paraxial wave theory. IEEE J. Quantum Electron. 40, 420–426 (2004).
Fiore, A., Bevilacqua, C. & Scarcelli, G. Direct three-dimensional measurement of refractive index via dual photon-phonon scattering. Phys. Rev. Lett. 122, 103901 (2019).
Caponi, S., Fioretto, D. & Mattarelli, M. On the actual spatial resolution of Brillouin Imaging. Opt. Lett. 45, 1063–1066 (2020).
Jacques, S. L. Optical properties of biological tissues: a review. Phys. Med. Biol. 58, R37 (2013).
Scarcelli, G. & Yun, S. H. In vivo Brillouin optical microscopy of the human eye. Opt. Express 20, 9197–9202 (2012).
Shao, P. et al. Effects of corneal hydration on brillouin microscopy in vivo. Investig. Ophthalmol. Vis. Sci. 59, 3020–3027 (2018).
Akilbekova, D. et al. Brillouin spectroscopy and radiography for assessment of viscoelastic and regenerative properties of mammalian bones. J. Biomed. Opt. 23, 097004 (2018).
Cardinali, M. et al. Brillouin micro-spectroscopy of subchondral, trabecular bone and articular cartilage of the human femoral head. Biomed. Opt. Express 10, 2606–2611 (2019).
Antonacci, G., Foreman, M. R., Paterson, C. & Török, P. Spectral broadening in Brillouin imaging. Apply. Phys. Lett. 103, 221105 (2013).
Ballmann, C. W., Meng, Z. & Yakovlev, V. V. Nonlinear Brillouin spectroscopy: what makes it a better tool for biological viscoelastic measurements. Biomed. Opt. Express 10, 1750–1759 (2019).
Caliari, S. R. & Burdick, J. A. A practical guide to hydrogels for cell culture. Nat. Methods 13, 405–414 (2016).
Acknowledgements
The authors thank M. Nikolic and A. Fiore for helpful discussions, and H. Zhang and E. Frank for helping with the LabVIEW program. This work was supported in part by the National Institutes of Health (K25HD097288, R33CA204582, U01CA202177, R01EY028666 and R01HD095520) and the National Science Foundation (CMMI 1929412 and DBI 1942003).
Author information
Authors and Affiliations
Contributions
Both authors conceived the idea; J.Z. performed the experiments; both authors wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
G.S holds patents related to Brillouin technology (US7898656B2, US8115919B2 and US20200278250A1) and is a consultant for Intelon Optics. The other authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Robert Prevedel, Vladislav Yakovlev, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol:
Scarcelli, G. et al. Nat. Methods 12, 1132-1134 (2015): https://doi.org/10.1038/nmeth.3616
Wisniewski, E. O. et al. Sci. Adv. 6, eaba6505 (2020): https://doi.org/10.1126/sciadv.aba6505
Zhang, J. et al. Small 16, 1907688 (2020): https://doi.org/10.1002/smll.201907688
Supplementary information
Supplementary Information
Supplementary Figs. 1–4 and Data 2–4.
Supplementary Data 1
MATLAB codes and example data.
Source data
Source Data Fig. 8
Raw data.
Source Data Fig. 9
Raw data.
Rights and permissions
About this article
Cite this article
Zhang, J., Scarcelli, G. Mapping mechanical properties of biological materials via an add-on Brillouin module to confocal microscopes. Nat Protoc 16, 1251–1275 (2021). https://doi.org/10.1038/s41596-020-00457-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-00457-2
This article is cited by
-
Brillouin microscopy monitors rapid responses in subcellular compartments
PhotoniX (2024)
-
Progressive alteration of murine bladder elasticity in actinic cystitis detected by Brillouin microscopy
Scientific Reports (2024)
-
Brillouin microscopy
Nature Reviews Methods Primers (2024)
-
Rapid biomechanical imaging at low irradiation level via dual line-scanning Brillouin microscopy
Nature Methods (2023)
-
Time-lapse mechanical imaging of neural tube closure in live embryo using Brillouin microscopy
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.