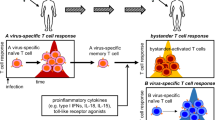
Abstract
During microbial infection, pre-existing memory CD8+ T cells that are not specific for the infecting pathogens can be activated by cytokines without cognate antigens, termed bystander activation. Studies in mouse models and human patients demonstrate bystander activation of memory CD8+ T cells, which exerts either protective or detrimental effects on the host, depending on the infection model or disease. Research has elucidated mechanisms underlying the bystander activation of CD8+ T cells in terms of the responsible cytokines and the effector mechanisms of bystander-activated CD8+ T cells. In this Review, we describe the history of research on bystander CD8+ T cell activation as well as evidence of bystander activation. We also discuss the mechanisms and immunopathological roles of bystander activation in various microbial infections.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Zhang, N. & Bevan, M. J. CD8+ T cells: foot soldiers of the immune system. Immunity 35, 161–168 (2011).
Whiteside, S. K., Snook, J. P., Williams, M. A. & Weis, J. J. Bystander T cells: a balancing act of friends and foes. Trends Immunol. 39, 1021–1035 (2018).
Simoni, Y. et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557, 575–579 (2018).
Kim, T. S. & Shin, E. C. The activation of bystander CD8+ T cells and their roles in viral infection. Exp. Mol. Med. 51, 1–9 (2019).
Lee, H. G., Cho, M. Z. & Choi, J. M. Bystander CD4+ T cells: crossroads between innate and adaptive immunity. Exp. Mol. Med. 52, 1255–1263 (2020).
Tough, D. F., Borrow, P. & Sprent, J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science 272, 1947–1950 (1996). This is among the earliest demonstrations of memory CD8+ T cell bystander activation that is induced by type I interferons.
Zhang, X., Sun, S., Hwang, I., Tough, D. F. & Sprent, J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8, 591–599 (1998).
Zarozinski, C. C. & Welsh, R. M. Minimal bystander activation of CD8 T cells during the virus-induced polyclonal T cell response. J. Exp. Med. 185, 1629–1639 (1997).
Ehl, S., Hombach, J., Aichele, P., Hengartner, H. & Zinkernagel, R. M. Bystander activation of cytotoxic T cells: studies on the mechanism and evaluation of in vivo significance in a transgenic mouse model. J. Exp. Med. 185, 1241–1251 (1997).
Murali-Krishna, K. et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8, 177–187 (1998). References 8–10 are early reports demonstrating bystander-activated memory CD8+ T cells that showed limited biological significance in mouse models of LCMV infection.
Chen, H. D. et al. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat. Immunol. 2, 1067–1076 (2001).
Brehm, M. A. et al. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat. Immunol. 3, 627–634 (2002).
Welsh, R. M. & Selin, L. K. No one is naive: the significance of heterologous T-cell immunity. Nat. Rev. Immunol. 2, 417–426 (2002).
Rehermann, B. & Shin, E. C. Private aspects of heterologous immunity. J. Exp. Med. 201, 667–670 (2005). References 13 and 14 are detailed reviews on CD8+ T cell cross-reactivity.
Lertmemongkolchai, G., Cai, G., Hunter, C. A. & Bancroft, G. J. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-γ in response to bacterial pathogens. J. Immunol. 166, 1097–1105 (2001).
Raue, H. P., Brien, J. D., Hammarlund, E. & Slifka, M. K. Activation of virus-specific CD8+ T cells by lipopolysaccharide-induced IL-12 and IL-18. J. Immunol. 173, 6873–6881 (2004).
Beadling, C. & Slifka, M. K. Differential regulation of virus-specific T-cell effector functions following activation by peptide or innate cytokines. Blood 105, 1179–1186 (2005).
Raue, H. P., Beadling, C., Haun, J. & Slifka, M. K. Cytokine-mediated programmed proliferation of virus-specific CD8+ memory T cells. Immunity 38, 131–139 (2013).
Ge, C. et al. Bystander activation of pulmonary Trm cells attenuates the severity of bacterial pneumonia by enhancing neutrophil recruitment. Cell Rep. 29, 4236–4244 (2019).
Berg, R. E., Crossley, E., Murray, S. & Forman, J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J. Exp. Med. 198, 1583–1593 (2003).
Freeman, B. E., Hammarlund, E., Raue, H. P. & Slifka, M. K. Regulation of innate CD8+ T-cell activation mediated by cytokines. Proc. Natl Acad. Sci. USA 109, 9971–9976 (2012).
Chu, T. et al. Bystander-activated memory CD8 T cells control early pathogen load in an innate-like, NKG2D-dependent manner. Cell Rep. 3, 701–708 (2013). This study reports that bystander-activated memory CD8+ T cells exert innate-like cytotoxicity contributing to early elimination of pathogens in a mouse model.
Sckisel, G. D. et al. Influenza infection results in local expansion of memory CD8+ T cells with antigen non-specific phenotype and function. Clin. Exp. Immunol. 175, 79–91 (2014).
Crosby, E. J., Goldschmidt, M. H., Wherry, E. J. & Scott, P. Engagement of NKG2D on bystander memory CD8 T cells promotes increased immunopathology following Leishmania major infection. PLoS Pathog. 10, e1003970 (2014). This study shows a detrimental role of bystander-activated CD8+ T cells recruited at the infection sites during mouse Leishmania infection.
Reynolds, J. M. & Dong, C. Toll-like receptor regulation of effector T lymphocyte function. Trends Immunol. 34, 511–519 (2013).
Salerno, F., Guislain, A., Cansever, D. & Wolkers, M. C. TLR-mediated innate production of IFN-γ by CD8+ T cells is independent of glycolysis. J. Immunol. 196, 3695–3705 (2016).
Whiteside, S. K. et al. IL-10 deficiency reveals a role for TLR2-dependent bystander activation of T cells in Lyme arthritis. J. Immunol. 200, 1457–1470 (2018).
Low, J. S. et al. Tissue-resident memory T cell reactivation by diverse antigen-presenting cells imparts distinct functional responses. J. Exp. Med. 217, e20192291 (2020).
Doisne, J. M. et al. CD8+ T cells specific for EBV, cytomegalovirus, and influenza virus are activated during primary HIV infection. J. Immunol. 173, 2410–2418 (2004).
Bastidas, S. et al. CD8+ T cells are activated in an antigen-independent manner in HIV-infected individuals. J. Immunol. 192, 1732–1744 (2014).
Younes, S. A. et al. IL-15 promotes activation and expansion of CD8+ T cells in HIV-1 infection. J. Clin. Invest. 126, 2745–2756 (2016). This study reports that proliferating memory CD8+ T cells during HIV-1 infection exhibit a highly diverse TCR repertoire.
Odumade, O. A. et al. Primary Epstein–Barr virus infection does not erode preexisting CD8+ T cell memory in humans. J. Exp. Med. 209, 471–478 (2012).
Ely, K. H. et al. Nonspecific recruitment of memory CD8+ T cells to the lung airways during respiratory virus infections. J. Immunol. 170, 1423–1429 (2003).
Tuuminen, T. et al. Human CD8+ T cell memory generation in Puumala hantavirus infection occurs after the acute phase and is associated with boosting of EBV-specific CD8+ memory T cells. J. Immunol. 179, 1988–1995 (2007).
Kohlmeier, J. E., Cookenham, T., Roberts, A. D., Miller, S. C. & Woodland, D. L. Type I interferons regulate cytolytic activity of memory CD8+ T cells in the lung airways during respiratory virus challenge. Immunity 33, 96–105 (2010).
Piet, B. et al. CD8+ T cells with an intraepithelial phenotype upregulate cytotoxic function upon influenza infection in human lung. J. Clin. Invest. 121, 2254–2263 (2011).
Lindgren, T. et al. Longitudinal analysis of the human T cell response during acute hantavirus infection. J. Virol. 85, 10252–10260 (2011).
Port, J. R. et al. Severe human Lassa fever is characterized by nonspecific T-cell activation and lymphocyte homing to inflamed tissues. J. Virol. 94, e01367-20 (2020).
Sandalova, E. et al. Contribution of herpesvirus specific CD8 T cells to anti-viral T cell response in humans. PLoS Pathog. 6, e1001051 (2010).
Shin, E. C., Sung, P. S. & Park, S. H. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat. Rev. Immunol. 16, 509–523 (2016).
Maini, M. K. et al. The role of virus-specific CD8+ cells in liver damage and viral control during persistent hepatitis B virus infection. J. Exp. Med. 191, 1269–1280 (2000).
Alanio, C. et al. Bystander hyperactivation of preimmune CD8+ T cells in chronic HCV patients. Elife 4, e07916 (2015).
Herndler-Brandstetter, D. et al. Post-thymic regulation of CD5 levels in human memory T cells is inversely associated with the strength of responsiveness to interleukin-15. Hum. Immunol. 72, 627–631 (2011).
Zhou, Y. et al. Dominance of the CD4+ T helper cell response during acute resolving hepatitis A virus infection. J. Exp. Med. 209, 1481–1492 (2012).
Lemon, S. M., Ott, J. J., Van Damme, P. & Shouval, D. Type A viral hepatitis: A summary and update on the molecular virology, epidemiology, pathogenesis and prevention. J. Hepatol. 68, 167–184 (2017).
Kim, J. et al. Innate-like cytotoxic function of bystander-activated CD8+ T cells is associated with liver injury in acute hepatitis A. Immunity 48, 161–173 (2018). This study demonstrates TCR-independent IL-15-induced activation of bystander memory CD8+ T cells that exert NKG2D-dependent cytotoxicity during acute HAV infection.
Miller, J. D. et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity 28, 710–722 (2008).
Seo, I. H. et al. IL-15 enhances CCR5-mediated migration of memory CD8. T cells by upregulating CCR5 expression in the absence of TCR stimulation. Cell. Rep. 36, 109438 (2021). This study demonstrates upregulation of CCR5 by IL-15 mediates the migration of bystander-activated memory CD8. T cells.
Weng, N. P., Liu, K., Catalfamo, M., Li, Y. & Henkart, P. A. IL-15 is a growth factor and an activator of CD8 memory T cells. Ann. N. Y. Acad. Sci. 975, 46–56 (2002).
Geginat, J., Lanzavecchia, A. & Sallusto, F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood 101, 4260–4266 (2003).
Setoguchi, R. IL-15 boosts the function and migration of human terminally differentiated CD8+ T cells by inducing a unique gene signature. Int. Immunol. 28, 293–305 (2016).
Waldmann, T. A. & Tagaya, Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu. Rev. Immunol. 17, 19–49 (1999).
Waldmann, T. A. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 6, 595–601 (2006).
Jabri, B. & Abadie, V. IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction. Nat. Rev. Immunol. 15, 771–783 (2015).
Mao, Y. et al. IL-15 activates mTOR and primes stress-activated gene expression leading to prolonged antitumor capacity of NK cells. Blood 128, 1475–1489 (2016).
Deshpande, P. et al. IL-7- and IL-15-mediated TCR sensitization enables T cell responses to self-antigens. J. Immunol. 190, 1416–1423 (2013).
Richer, M. J. et al. Inflammatory IL-15 is required for optimal memory T cell responses. J. Clin. Invest. 125, 3477–3490 (2015).
Liu, K., Catalfamo, M., Li, Y., Henkart, P. A. & Weng, N. P. IL-15 mimics T cell receptor crosslinking in the induction of cellular proliferation, gene expression, and cytotoxicity in CD8+ memory T cells. Proc. Natl Acad. Sci. USA 99, 6192–6197 (2002).
Correia, M. P. et al. Hepatocytes and IL-15: a favorable microenvironment for T cell survival and CD8+ T cell differentiation. J. Immunol. 182, 6149–6159 (2009).
Correia, M. P., Costa, A. V., Uhrberg, M., Cardoso, E. M. & Arosa, F. A. IL-15 induces CD8+ T cells to acquire functional NK receptors capable of modulating cytotoxicity and cytokine secretion. Immunobiology 216, 604–612 (2011).
Balin, S. J. et al. Human antimicrobial cytotoxic T lymphocytes, defined by NK receptors and antimicrobial proteins, kill intracellular bacteria. Sci. Immunol. 3, eaat7668 (2018).
Ostler, T., Pircher, H. & Ehl, S. ‘Bystander’ recruitment of systemic memory T cells delays the immune response to respiratory virus infection. Eur. J. Immunol. 33, 1839–1848 (2003).
Crosby, E. J., Clark, M., Novais, F. O., Wherry, E. J. & Scott, P. Lymphocytic choriomeningitis virus expands a population of NKG2D+CD8+ T cells that exacerbates disease in mice coinfected with Leishmania major. J. Immunol. 195, 3301–3310 (2015).
Soudja, S. M., Ruiz, A. L., Marie, J. C. & Lauvau, G. Inflammatory monocytes activate memory CD8+ T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity 37, 549–562 (2012). This study describes two major cytokines, IL-15 and IL-18, that induce bystander activation of memory CD8+ T cells.
Bauer, S. et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285, 727–729 (1999).
Roberts, A. I. et al. NKG2D receptors induced by IL-15 costimulate CD28-negative effector CTL in the tissue microenvironment. J. Immunol. 167, 5527–5530 (2001).
Raulet, D. H. Roles of the NKG2D immunoreceptor and its ligands. Nat. Rev. Immunol. 3, 781–790 (2003).
Groh, V. et al. Costimulation of CD8αβ T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat. Immunol. 2, 255–260 (2001).
Verneris, M. R., Karimi, M., Baker, J., Jayaswal, A. & Negrin, R. S. Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood 103, 3065–3072 (2004).
Meresse, B. et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity 21, 357–366 (2004).
Green, P. H. & Cellier, C. Celiac disease. N. Engl. J. Med. 357, 1731–1743 (2007).
Hue, S. et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 21, 367–377 (2004).
Okamura, H. et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature 378, 88–91 (1995).
Nakanishi, K., Yoshimoto, T., Tsutsui, H. & Okamura, H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 12, 53–72 (2001).
Hunter, C. A. et al. Comparison of the effects of interleukin-1α, interleukin-1β and interferon-γ-inducing factor on the production of interferon-γ by natural killer. Eur. J. Immunol. 27, 2787–2792 (1997).
Tomura, M. et al. A critical role for IL-18 in the proliferation and activation of NK1.1+CD3− cells. J. Immunol. 160, 4738–4746 (1998).
Kambayashi, T., Assarsson, E., Lukacher, A. E., Ljunggren, H. G. & Jensen, P. E. Memory CD8+ T cells provide an early source of IFN-γ. J. Immunol. 170, 2399–2408 (2003).
Smeltz, R. B. Profound enhancement of the IL-12/IL-18 pathway of IFN-γ secretion in human CD8+ memory T cell subsets via IL-15. J. Immunol. 178, 4786–4792 (2007).
Maurice, N. J., McElrath, M. J., Andersen-Nissen, E., Frahm, N. & Prlic, M. CXCR3 enables recruitment and site-specific bystander activation of memory CD8+ T cells. Nat. Commun. 10, 4987 (2019).
Verbist, K. C., Cole, C. J., Field, M. B. & Klonowski, K. D. A role for IL-15 in the migration of effector CD8 T cells to the lung airways following influenza infection. J. Immunol. 186, 174–182 (2011).
Sowell, R. T. et al. IL-15 complexes induce migration of resting memory CD8 T cells into mucosal tissues. J. Immunol. 199, 2536–2546 (2017).
Nolz, J. C. & Harty, J. T. IL-15 regulates memory CD8+ T cell O-glycan synthesis and affects trafficking. J. Clin. Invest. 124, 1013–1026 (2014).
Martin, M. D. & Badovinac, V. P. Antigen-dependent and -independent contributions to primary memory CD8 T cell activation and protection following infection. Sci. Rep. 5, 18022 (2015).
Nikolich-Zugich, J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat. Rev. Immunol. 8, 512–522 (2008).
Mittelbrunn, M. & Kroemer, G. Hallmarks of T cell aging. Nat. Immunol. 22, 687–698 (2021).
Khan, N. et al. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J. Immunol. 173, 7481–7489 (2004).
Griffiths, S. J. et al. Age-associated increase of low-avidity cytomegalovirus-specific CD8+ T cells that re-express CD45RA. J. Immunol. 190, 5363–5372 (2013).
Chiu, W. K., Fann, M. & Weng, N. P. Generation and growth of CD28nullCD8+ memory T cells mediated by IL-15 and its induced cytokines. J. Immunol. 177, 7802–7810 (2006).
Morris, S. R. et al. Inflammescent CX3CR1+CD57+CD8+ T cells are generated and expanded by IL-15. JCI Insight 5, e132963 (2020).
Beura, L. K. et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532, 512–516 (2016). This study introduces mouse models that better recapitulate the immune system of human adults in terms of the memory-pool size.
White, J. T., Cross, E. W. & Kedl, R. M. Antigen-inexperienced memory CD8+ T cells: where they come from and why we need them. Nat. Rev. Immunol. 17, 391–400 (2017).
Goldrath, A. W., Bogatzki, L. Y. & Bevan, M. J. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J. Exp. Med. 192, 557–564 (2000).
Sosinowski, T. et al. CD8α+ dendritic cell trans presentation of IL-15 to naive CD8+ T cells produces antigen-inexperienced T cells in the periphery with memory phenotype and function. J. Immunol. 190, 1936–1947 (2013).
Lee, J. Y., Hamilton, S. E., Akue, A. D., Hogquist, K. A. & Jameson, S. C. Virtual memory CD8 T cells display unique functional properties. Proc. Natl Acad. Sci. USA 110, 13498–13503 (2013).
Quinn, K. M. et al. Heightened self-reactivity associated with selective survival, but not expansion, of naive virus-specific CD8+ T cells in aged mice. Proc. Natl Acad. Sci. USA 113, 1333–1338 (2016).
White, J. T. et al. Virtual memory T cells develop and mediate bystander protective immunity in an IL-15-dependent manner. Nat. Commun. 7, 11291 (2016).
Jacomet, F. et al. Evidence for eomesodermin-expressing innate-like CD8+KIR/NKG2A+ T cells in human adults and cord blood samples. Eur. J. Immunol. 45, 1926–1933 (2015).
Quinn, K. M. et al. Metabolic characteristics of CD8+ T cell subsets in young and aged individuals are not predictive of functionality. Nat. Commun. 11, 2857 (2020).
Rolot, M. et al. Helminth-induced IL-4 expands bystander memory CD8+ T cells for early control of viral infection. Nat. Commun. 9, 4516 (2018).
Lin, J. S. et al. Virtual memory CD8 T cells expanded by helminth infection confer broad protection against bacterial infection. Mucosal Immunol. 12, 258–264 (2019).
Tan, J. T. et al. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 195, 1523–1532 (2002).
Akue, A. D., Lee, J. Y. & Jameson, S. C. Derivation and maintenance of virtual memory CD8 T cells. J. Immunol. 188, 2516–2523 (2012).
Haluszczak, C. et al. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J. Exp. Med. 206, 435–448 (2009).
Kjer-Nielsen, L. et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 491, 717–723 (2012).
Corbett, A. J. et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature 509, 361–365 (2014).
Godfrey, D. I., Uldrich, A. P., McCluskey, J., Rossjohn, J. & Moody, D. B. The burgeoning family of unconventional T cells. Nat. Immunol. 16, 1114–1123 (2015).
Legoux, F., Salou, M. & Lantz, O. Unconventional or preset αβ T cells: evolutionarily conserved tissue-resident T cells recognizing nonpeptidic ligands. Annu. Rev. Cell Dev. Biol. 33, 511–535 (2017).
van Wilgenburg, B. et al. MAIT cells are activated during human viral infections. Nat. Commun. 7, 11653 (2016).
Loh, L. et al. Human mucosal-associated invariant T cells contribute to antiviral influenza immunity via IL-18-dependent activation. Proc. Natl Acad. Sci. USA 113, 10133–10138 (2016).
Paquin-Proulx, D. et al. MAIT cells are activated in acute Dengue virus infection and after in vitro Zika virus infection. PLoS Negl. Trop. Dis. 12, e0006154 (2018).
Ussher, J. E. et al. CD161++CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12 + IL-18 in a TCR-independent manner. Eur. J. Immunol. 44, 195–203 (2014).
Jo, J. et al. Toll-like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLoS Pathog. 10, e1004210 (2014).
Rha, M. S. et al. Human liver CD8+ MAIT cells exert TCR/MR1-independent innate-like cytotoxicity in response to IL-15. J. Hepatol. 73, 640–650 (2020).
Chakir, H., Lam, D. K., Lemay, A. M. & Webb, J. R. ‘Bystander polarization’ of CD4+ T cells: activation with high-dose IL-2 renders naive T cells responsive to IL-12 and/or IL-18 in the absence of TCR ligation. Eur. J. Immunol. 33, 1788–1798 (2003).
Chung, Y. et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 30, 576–587 (2009).
Guo, L. et al. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc. Natl Acad. Sci. USA 106, 13463–13468 (2009).
Lee, Y. K. et al. TCR-independent functions of Th17 cells mediated by the synergistic actions of cytokines of the IL-12 and IL-1 families. PLoS ONE 12, e0186351 (2017).
Polley, R., Zubairi, S. & Kaye, P. M. The fate of heterologous CD4+ T cells during Leishmania donovani infection. Eur. J. Immunol. 35, 498–504 (2005).
Guo, L. et al. Innate immunological function of TH2 cells in vivo. Nat. Immunol. 16, 1051–1059 (2015).
Gangappa, S., Deshpande, S. P. & Rouse, B. T. Bystander activation of CD4+ T cells can represent an exclusive means of immunopathology in a virus infection. Eur. J. Immunol. 29, 3674–3682 (1999).
Gangappa, S., Deshpande, S. P. & Rouse, B. T. Bystander activation of CD4+ T cells accounts for herpetic ocular lesions. Invest. Ophthalmol. Vis. Sci. 41, 453–459 (2000).
Lee, H. G. et al. Pathogenic function of bystander-activated memory-like CD4+ T cells in autoimmune encephalomyelitis. Nat. Commun. 10, 709 (2019).
Judge, A. D., Zhang, X., Fujii, H., Surh, C. D. & Sprent, J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8+ T cells. J. Exp. Med. 196, 935–946 (2002).
Lu, J. et al. Interleukin 15 promotes antigen-independent in vitro expansion and long-term survival of antitumor cytotoxic T lymphocytes. Clin. Cancer Res. 8, 3877–3884 (2002).
Nakamura, R. et al. Interleukin-15 is critical in the pathogenesis of influenza a virus-induced acute lung injury. J. Virol. 84, 5574–5582 (2010).
Belkaya, S. et al. Inherited IL-18BP deficiency in human fulminant viral hepatitis. J. Exp. Med. 216, 1777–1790 (2019). This study reports immunopathological liver injury during acute HAV infection due to uncontrolled activity of IL-18.
Duhen, T. et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun. 9, 2724 (2018).
Scheper, W. et al. Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat. Med. 25, 89–94 (2019).
Tietze, J. K. et al. Delineation of antigen-specific and antigen-nonspecific CD8+ memory T-cell responses after cytokine-based cancer immunotherapy. Blood 119, 3073–3083 (2012).
Wong, H. C., Jeng, E. K. & Rhode, P. R. The IL-15-based superagonist ALT-803 promotes the antigen-independent conversion of memory CD8+ T cells into innate-like effector cells with antitumor activity. Oncoimmunology 2, e26442 (2013).
Erkes, D. A. et al. Virus-specific CD8+ T cells infiltrate melanoma lesions and retain function independently of PD-1 expression. J. Immunol. 198, 2979–2988 (2017).
Danahy, D. B., Berton, R. R. & Badovinac, V. P. Cutting edge: antitumor immunity by pathogen-specific CD8 T cells in the absence of cognate antigen recognition. J. Immunol. 204, 1431–1435 (2020).
Rosato, P. C. et al. Virus-specific memory T cells populate tumors and can be repurposed for tumor immunotherapy. Nat. Commun. 10, 567 (2019).
Newman, J. H. et al. Intratumoral injection of the seasonal flu shot converts immunologically cold tumors to hot and serves as an immunotherapy for cancer. Proc. Natl Acad. Sci. USA 117, 1119–1128 (2020).
Reese, T. A. et al. Sequential infection with common pathogens promotes human-like immune gene expression and altered vaccine response. Cell Host Microbe 19, 713–719 (2016).
Rosshart, S. P. et al. Laboratory mice born to wild mice have natural microbiota and model human immune responses. Science 365, eaaw4361 (2019).
Acknowledgements
We thank J. E. Oh (KAIST) for critical discussion. This work was supported by the Samsung Science and Technology Foundation under project number SSTF-BA1402-51.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Immunology thanks Antonio Bertoletti and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Zoltan Fehervari was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, H., Jeong, S. & Shin, EC. Significance of bystander T cell activation in microbial infection. Nat Immunol 23, 13–22 (2022). https://doi.org/10.1038/s41590-021-00985-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-021-00985-3
This article is cited by
-
Temperature impacts the bovine ex vivo immune response towards Mycoplasmopsis bovis
Veterinary Research (2024)
-
Characterization of tumoricidal activities mediated by a novel immune cell regimen composing interferon-producing killer dendritic cells and tumor-specific cytotoxic T lymphocytes
BMC Cancer (2024)
-
BCG immunization induces CX3CR1hi effector memory T cells to provide cross-protection via IFN-γ-mediated trained immunity
Nature Immunology (2024)
-
Immune and inflammatory mechanisms in hypertension
Nature Reviews Cardiology (2024)
-
Immunization-induced antigen archiving enhances local memory CD8+ T cell responses following an unrelated viral infection
npj Vaccines (2024)