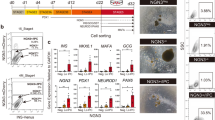
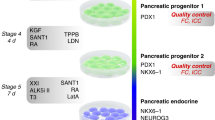
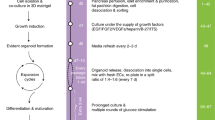
Abstract
Methods for differentiating human pluripotent stem cells to pancreatic and liver lineages in vitro have been limited by the inability to identify and isolate distinct endodermal subpopulations specific to these two organs. Here we report that pancreatic and hepatic progenitors can be isolated using the surface markers CD177/NB1 glycoprotein and inducible T-cell costimulatory ligand CD275/ICOSL, respectively, from seemingly homogeneous definitive endoderm derived from human pluripotent stem cells. Anterior definitive endoderm (ADE) subpopulations identified by CD177 and CD275 show inverse activation of canonical and noncanonical WNT signaling. CD177+ ADE expresses and synthesizes the secreted WNT, NODAL and BMP antagonist CERBERUS1 and is specified toward the pancreatic fate. CD275+ ADE receives canonical Wnt signaling and is specified toward the liver fate. Isolated CD177+ ADE differentiates more homogeneously into pancreatic progenitors and into more functionally mature and glucose-responsive β-like cells in vitro compared with cells from unsorted differentiation cultures.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Additional data supporting the findings of this study are available from the corresponding author upon request.
Code availability
All microarray data are available at GEO under the accession number GSE113791.
References
Zorn, A. M. & Wells, J. M. Vertebrate endoderm development and organ formation. Annu. Rev. Cell Dev. Biol. 25, 221–251 (2009).
Tremblay, K. D. & Zaret, K. S. Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev. Biol. 280, 87–99 (2005).
Tam, P. P. L. et al. Sequential allocation and global pattern of movement of the definitive endoderm in the mouse embryo during gastrulation. Development 134, 251–260 (2007).
Zaret, K. S. Genetic programming of liver and pancreas progenitors: lessons for stem-cell differentiation. Nat. Rev. Genet. 9, 329–340 (2008).
Serls, A. E., Doherty, S., Parvatiyar, P., Wells, J. M. & Deutsch, G. H. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development 132, 35–47 (2005).
Wang, Z., Dollé, P., Cardoso, W. V. & Niederreither, K. Retinoic acid regulates morphogenesis and patterning of posterior foregut derivatives. Dev. Biol. 297, 433–445 (2006).
Rodríguez-Seguel, E. et al. Mutually exclusive signaling signatures define the hepatic and pancreatic progenitor cell lineage divergence. Genes Dev. 27, 1932–1946 (2013).
Jennings, R. E. et al. Development of the human pancreas from foregut to endocrine commitment. Diabetes 62, 3514–3522 (2013).
D’Amour, K. A., Agulnick, A. D., Eliazer, S., Kelly, O. G. & Kroon, E. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 23, 1534–1541 (2005).
Rezania, A. et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 32, 1121–1133 (2014).
Pagliuca, F. W. et al. Generation of functional human pancreatic β cells in vitro. Cell 159, 428–439 (2014).
Russ, H. A. et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 34, 1759–1772 (2015).
Canham, M. A., Sharov, A. A., Ko, M. S. H. & Brickman, J. M. Functional heterogeneity of embryonic stem cells revealed through translational amplification of an early endodermal transcript. PLoS Biol. 8, e1000379 (2010).
Morrison, G. M. et al. Anterior definitive endoderm from ESCs reveals a role for FGF signaling. Cell Stem Cell 3, 402–415 (2008).
Gadue, P. et al. Generation of monoclonal antibodies specific for cell surface molecules expressed on early mouse endoderm. Stem Cells 27, 2103–2113 (2009).
Kelly, O. G. et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat. Biotechnol. 29, 750–756 (2011).
Ameri, J. et al. Efficient generation of glucose-responsive beta cells from isolated GP2+ human pancreatic progenitors. Cell Rep. 19, 36–49 (2017).
Cogger, K. F. et al. Glycoprotein 2 is a specific cell surface marker of human pancreatic progenitors. Nat. Commun. 8, 331 (2017).
Burtscher, I. & Lickert, H. Foxa2 regulates polarity and epithelialization in the endoderm germ layer of the mouse embryo. Development 136, 1029–1038 (2009).
Deglincerti, A. et al. Self-organization of the in vitro attached human embryo. Nature 533, 251–254 (2016).
Hoshino, H., Shioi, G. & Aizawa, S. AVE protein expression and visceral endoderm cell behavior during anterior–posterior axis formation in mouse embryos: asymmetry in OTX2 and DKK1 expression. Dev. Biol. 402, 175–191 (2015).
Stroncek, D. F. Neutrophil-specific antigen HNA-2a, NB1 glycoprotein, and CD177. Curr. Opin. Hematol. 14, 688–693 (2007).
Hu, H. et al. Noncanonical NF-κB regulates inducible costimulator (ICOS) ligand expression and T follicular helper cell development. Proc. Natl Acad. Sci. USA 108, 12827–12832 (2011).
Rezania, A. et al. Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo. Stem Cells 31, 2432–2442 (2013).
Xu, X., Browning, V. & Odorico, J. Activin, BMP and FGF pathways cooperate to promote endoderm and pancreatic lineage cell differentiation from human embryonic stem cells. Mech. Dev. 128, 412–427 (2011).
Yap, C., Goh, H. N., Familari, M., Rathjen, P. D. & Rathjen, J. The formation of proximal and distal definitive endoderm populations in culture requires p38 MAPK activity. J. Cell Sci. 127, 2204–2216 (2014).
Piccolo, S. et al. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature 397, 707–710 (1999).
MacDonald, B. T., Tamai, K. & He, X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 (2009).
Chen, Y.-F. et al. Rapid generation of mature hepatocyte-like cells from human induced pluripotent stem cells by an efficient three-step protocol. Hepatology 55, 1193–1203 (2012).
Decker, K., Goldman, D. C., Grasch, C. L. & Sussel, L. Gata6 is an important regulator of mouse pancreas development. Dev. Biol. 298, 415–429 (2006).
Tiyaboonchai, A. et al. GATA6 plays an important role in the induction of human definitive endoderm, development of the pancreas, and functionality of pancreatic β cells. Stem Cell Reports 8, 589–604 (2017).
Chen, B. et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 5, 100–107 (2009).
Pei, Y. et al. WNT signaling increases proliferation and impairs differentiation of stem cells in the developing cerebellum. Development 139, 1724–1733 (2012).
Bader, E. et al. Identification of proliferative and mature β-cells in the islets of Langerhans. Nature 535, 430–434 (2016).
Johansson, K. A. et al. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev. Cell 12, 457–465 (2007).
Krentz, N. A. J. et al. Phosphorylation of NEUROG3 links endocrine differentiation to the cell cycle in pancreatic progenitors. Dev. Cell 41, 129–142 e126 (2017).
Roscioni, S. S., Migliorini, A., Gegg, M. & Lickert, H. Impact of islet architecture on β-cell heterogeneity, plasticity and function. Nat. Rev. Endocrinol. 12, 695–709 (2016).
Vincent, S. D., Dunn, N. R., Hayashi, S., Norris, D. P. & Robertson, E. J. Cell fate decisions within the mouse organizer are governed by graded Nodal signals. Genes Dev. 17, 1646–1662 (2003).
Perea-Gomez, A. et al. Nodal antagonists in the anterior visceral endoderm prevent the formation of multiple primitive streaks. Dev. Cell 3, 745–756 (2002).
Arnold, S. J. & Robertson, E. J. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat. Rev. Mol. Cell Biol. 10, 91–103 (2009).
Aykul, S., Ni, W., Mutatu, W. & Martinez-Hackert, E. Human cerberus prevents Nodal-receptor binding, inhibits Nodal signaling, and suppresses Nodal-mediated phenotypes. PLoS ONE 10, e0114954 (2015).
Cortijo, C., Gouzi, M., Tissir, F. & Grapin-Botton, A. Planar cell polarity controls pancreatic beta cell differentiation and glucose homeostasis. Cell Rep. 2, 1593–1606 (2012).
Pezzulo, A. A. et al. The air-liquid interface and use of primary cell cultures are important to recapitulate the transcriptional profile of in vivo airway epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 300, L25–L31 (2011).
Kim, Y. et al. Islet-like organoids derived from human pluripotent stem cells efficiently function in the glucose responsiveness in vitro and in vivo. Sci. Rep. 6, 35145 (2016).
Millman, J. R. et al. Generation of stem cell-derived β-cells from patients with type 1 diabetes. Nat. Commun. 7, 11463 (2016).
Velazco-Cruz, L. et al. Acquisition of dynamic function in human stem cell-derived β cells. Stem Cell Reports 12, 351–365 (2019).
Nair, G. G. et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived β cells. Nat. Cell Biol. 21, 263–274 (2019).
Cheng, X. et al. Self-renewing endodermal progenitor lines generated from human pluripotent stem cells. Cell Stem Cell 10, 371–384 (2012).
Wang, X. et al. Generation of a human induced pluripotent stem cell (iPSC) line from a patient with family history of diabetes carrying a C18R mutation in the PDX1 gene. Stem Cell Res. 17, 292–295 (2016).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Acknowledgements
We thank A. Rezania for providing hMAFA and hGLUT1 antibodies. We thank the Alberta Diabetes Institute IsletCore and Olle Korsgren from Uppsala University for human islets. We are grateful to I. Burtscher, A. Bastidas-Ponce, C. Salinno and M. Tarquis-Medina for their invaluable inputs and technical assistance. We thank D. M. Thomson and M. Catani for reading and correction of the manuscript. This work was supported by the HumEN consortium funded by European Union’s Seventh Framework Programme for Research, Technological Development and Demonstration under grant agreement no. 602889 (http://www.hum-en.eu/). This project has received funding from the European Union’s Horizon 2020 researchand innovation programme under grant agreement no. 874839. This work was also funded in part by the German Center for Diabetes Research (DZD e.V.) and the Helmholtz Alliance ‘Aging and Metabolic Programming, AMPro’. P.U.M. was funded by a postdoctoral grant from the HumEN consortium, EU. M.S., S.P. and K.S. are supported by the Helena Graduate School and Technische Universität München. S.K. is an employee of Miltenyi Biotec GmbH, Bergisch Gladbach, Germany. The project was funded by the German Federal Ministry of Education and Research (project number 01EK1607A).
Author information
Authors and Affiliations
Contributions
Sandra Pfluger and Ansarullah contributed equally to this work. P.U.M. and H.L. wrote the manuscript. P.U.M. and H.L. designed experiments, and analyzed and interpreted the results. K.S., S.P. and A. performed experiments and analyzed data. M.S. provided bioinformatics assistance and analyzed the results. J. Beckenbauer provided technical assistance. S.K. designed, performed and analyzed the antibody screen. J. Beckers and M.I. performed and analyzed the microarray chips. P.U.M. and H.L. conceived the project.
Corresponding author
Ethics declarations
Competing interests
Helmholtz Zentrum München and Miltenyi Biotec own a patent (International Application no. PCT/EP2018/065779), ‘Methods for purifying endoderm and pancreatic endoderm cells derived from human embryonic stem cells’, using CD177 and CD275 antibodies for enrichment or depletion of pancreatic and liver progenitors).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Screening strategy for the identification of endoderm subpopulations.
(a) Screening work flow for the initial screen. (b-d) Representative FACS plots for CD177 and CD275 (b) labelling of differentiated day 4 DE cells with known endoderm markers (FOXA2 and CXCR4) revealed definitive endoderm (FOXA2+/CXCR4+) and mes-endoderm (FOXA2low/CXCR4-) subpopulations. (c-d) CD177 and CD275 expression profiles reveal different endoderm subpopulations (n = 1). (e) Immunofluorescent staining for CER1 with FOXA2 in DE cultures (n = 3, biologically independent experiments). Scale bar, 25 µm. (f) FACS analysis for CD275+/CER1+ and CD177+/CER1+ ADE cell populations at day 4 DE. (g-h) qPCR quantification for the mRNA expression of FOXA2 and SOX17 (g), CER1 and HHEX (h) in enriched CD177+ and CD275+ ADE subpopulations (ANOVA, (e-h) n = 3, biologically independent experiments). Data is represented as mean ±s.e.m; P<0.05 and P<0.01. Statistically non-significant results are not indicated in the figure.
Supplementary Figure 2 Percentage of CD177+ and CD275+ ADE subpopulations induced in different hESC and hiPSC lines.
(a) Endoderm differentiation scheme from hESCs toward DE/ADE. (b) FACS plots represent the percentage of CXCR4+/CD177+ and CXCR4+/CD275+ subpopulations in hH1, hMEL1-NKX6.1, HMGUi001-A hiPSC, HUES8 and H9 at DE/ADE stage. (c) Quantification of flow cytometry data from ((b,c) n = 3, biologically independent experiments).
Supplementary Figure 3 Induction efficiency of CD177+ and CD275+ ADE shows variation using different protocols.
(a-c) Adaptation of previously published endoderm differentiation protocols from hESCs. (d) FACS quantification for the percentage of total population expressing CXCR4 in DE cells derived from HMGUi001-A hiPSC using 3 different endoderm induction protocols (n = 2 biologically independent experiments). (e) FACS quantification for the percentage of cells expressing CXCR4+/CD177+ and CXCR4+/CD275+ in DE generated using previously published protocols (n = 3 (HMGUi001-A hiPSC), n = 2 (H9) biologically independent experiments). Data is represented as mean ±s.e.m.
Supplementary Figure 4 Differentiation of enriched CD177+, CD275+ and CXCR4+ ADE subpopulations toward liver and pancreas fate.
(a) Expression of CD177-, CD275-, and CXCR4 during differentiation of hESCs toward pancreatic β-like cells. (b) Liver differentiation protocol. (c) qPCR quantification of the expression of early liver progenitor markers HHEX, TTR and AFP in enriched ADE subpopulations (ANOVA, n = 3, biologically independent experiments). (d) Immunofluorescent staining of pancreatic progenitor cells derived from enriched ADE subpopulations for the co-expression of posterior foregut marker GATA6 and PDX1. (e) Immunofluorescent staining of pancreatic progenitor cells derived from enriched ADE subpopulations for the co-expression of lung marker SOX2, intestinal marker CDX2 and PDX1 ((d, e) n = 3, biologically independent experiments). Data is represented as mean ±s.e.m; P<0.05 and P<0.01. Statistically non-significant results are not indicated in the figure. Scale bars, 25µm.
Supplementary Figure 5 CD177+ ADE positively correlates with PP1 induction.
(a) Pancreatic induction protocol. (b) FACS analysis of H1, HMGUi001-A hiPSC, HUES8 and MEL1-NKX6.1 for PDX1 at S3 stage. (c) Quantification of CD177+ cells generated at S1 and PDX1+ cells generated at S3 stage showing correlation between CD177 and PDX1 induction (n = 10 (H1), n = 7 (MEL1-NKX6.1), n = 9 (HMGUi001-A), n = 9 (HuES8), n = 8 (H9)). Data is represented as mean ±s.e.m.
Supplementary Figure 6 H1 hESC pancreatic and endocrine differentiations of CD177+- and US-DE.
(a) Overview of differentiation protocol used to generate CD177-/US-β-cells. (b-e) Immunostainings for INS and NKX6.1 (b), GCG, INS and SST (c) and C-peptide and GLUT1 (d), INS, MAFA and NKX6.1 (e) in CD177- and US- β-cells ((b-e) n = 3, biologically independent experiments). Scale bars, 50 µm. (f) Representative flow cytometry contour plots of S4 and S7 cells generated from CD177+- and US-ADE/DE cells on H1 line and stained for indicated markers. (g,h) Percentage of cells expressing indicated markers (two-sided unpaired Student’s t-test, (g) n = 3, (h) n=13, n = 17 for INS+, biologically independent experiments). Data is represented as mean ±s.e.m; P<0.05 and P<0.01. Statistically non-significant results are not indicated in the figure.
Supplementary Figure 7 Comparison of 2D and 3D culture system on pancreatic differentiation.
(a) Overview of differentiation protocol used. (b) Comparison of PDX1+/NKX6.1+ generated from CD177+- and CXCR4+-ADE in 2D and 3D settings (two-sided unpaired Student’s t-test, n = 3 biologically independent experiments). (c) Morphology of CD177- and CXCR4-β-cells; DAPI (blue) and E-CAD (green). Scale bars, 20 µm. Graph represents the size of the aggregates in µm (two-sided unpaired Student’s t-test, n = 15 aggregates from 3 biologically independent experiments). Data is represented as mean ±s.e.m, P<0.05. Statistically non-significant results are not indicated in the figure.
Supplementary Figure 8 H1 hESC-derived CD177+-ADE generates more functional β-like cells in vitro.
(a) Insulin content of US-β-cells and CD177-β-cells (two-sided unpaired Student’s t-test, n = 10, biologically independent experiments). (b) Comparison of insulin secretion of US-β-cells and CD177-β-cells in sequential static GSIS (two-sided unpaired Student’s t-test, n = 5, biologically independent experiments). (c,d) Insulin secretion in response to dynamic glucose, Ex-4 and KCl challenges in a perifusion system on US-β-cells (c) and CD177-β-cells (d) ((c,d) n = 5, biologically independent experiments). Data is represented as mean ±s.e.m; P<0.05 and P<0.01. Statistically non-significant results are not indicated in the.
Supplementary Figure 9 CD177+ and CD275+ ADE receive differential Wnt signaling.
(a,b) Original Western blot of WNT/PCP components such as DVL2 (a) and p-JNK (b) in ADE subpopulations as shown in Fig. 2h.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9 and Tables 1–4.
Rights and permissions
About this article
Cite this article
Mahaddalkar, P.U., Scheibner, K., Pfluger, S. et al. Generation of pancreatic β cells from CD177+ anterior definitive endoderm. Nat Biotechnol 38, 1061–1072 (2020). https://doi.org/10.1038/s41587-020-0492-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-020-0492-5
This article is cited by
-
Metabolic switching, growth kinetics and cell yields in the scalable manufacture of stem cell-derived insulin-producing cells
Stem Cell Research & Therapy (2024)
-
From stem cells to pancreatic β-cells: strategies, applications, and potential treatments for diabetes
Molecular and Cellular Biochemistry (2024)
-
Single-nucleus multi-omics of human stem cell-derived islets identifies deficiencies in lineage specification
Nature Cell Biology (2023)
-
Stepwise differentiation of functional pancreatic β cells from human pluripotent stem cells
Cell Regeneration (2022)
-
N6-methyladenosine modification-mediated mRNA metabolism is essential for human pancreatic lineage specification and islet organogenesis
Nature Communications (2022)