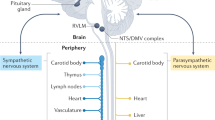
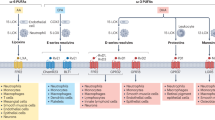
Abstract
Atherosclerotic plaques develop in the inner intimal layer of arteries and can cause heart attacks and strokes1. As plaques lack innervation, the effects of neuronal control on atherosclerosis remain unclear. However, the immune system responds to plaques by forming leukocyte infiltrates in the outer connective tissue coat of arteries (the adventitia)2,3,4,5,6. Here, because the peripheral nervous system uses the adventitia as its principal conduit to reach distant targets7,8,9, we postulated that the peripheral nervous system may directly interact with diseased arteries. Unexpectedly, widespread neuroimmune cardiovascular interfaces (NICIs) arose in mouse and human atherosclerosis-diseased adventitia segments showed expanded axon networks, including growth cones at axon endings near immune cells and media smooth muscle cells. Mouse NICIs established a structural artery–brain circuit (ABC): abdominal adventitia nociceptive afferents10,11,12,13,14 entered the central nervous system through spinal cord T6–T13 dorsal root ganglia and were traced to higher brain regions, including the parabrachial and central amygdala neurons; and sympathetic efferent neurons projected from medullary and hypothalamic neurons to the adventitia through spinal intermediolateral neurons and both coeliac and sympathetic chain ganglia. Moreover, ABC peripheral nervous system components were activated: splenic sympathetic and coeliac vagus nerve activities increased in parallel to disease progression, whereas coeliac ganglionectomy led to the disintegration of adventitial NICIs, reduced disease progression and enhanced plaque stability. Thus, the peripheral nervous system uses NICIs to assemble a structural ABC, and therapeutic intervention in the ABC attenuates atherosclerosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Microarray data were deposited at the NCBI Gene Expression Omnibus (GSE94044 (adventitia), GSE93954 (ganglia), GSE40156 (aorta, spleen and RLN)). The script for data analyses of electrophysiological recordings has been previously published32 and is available at https://github.com/LorCarnevale/CVNA_Process. All other relevant data are available from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Libby, P., Ridker, P. M. & Hansson, G. K. Progress and challenges in translating the biology of atherosclerosis. Nature 473, 317–325 (2011).
Mohanta, S. K. et al. Artery tertiary lymphoid organs contribute to innate and adaptive immune responses in advanced mouse atherosclerosis. Circ. Res. 114, 1772–1787 (2014).
Grabner, R. et al. Lymphotoxin beta receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE-/- mice. J. Exp. Med. 206, 233–248 (2009).
Hu, D. et al. Artery tertiary lymphoid organs control aorta immunity and protect against atherosclerosis via vascular smooth muscle cell lymphotoxin β receptors. Immunity 42, 1100–1115 (2015).
Akhavanpoor, M. et al. Adventitial inflammation and its interaction with intimal atherosclerotic lesions. Front. Physiol. 5, 296 (2014).
Yin, C., Mohanta, S. K., Srikakulapu, P., Weber, C. & Habenicht, A. J. Artery tertiary lymphoid organs: powerhouses of atherosclerosis immunity. Frontiers in Immunol. 7, 387 (2016).
Mukouyama, Y. S., Shin, D., Britsch, S., Taniguchi, M. & Anderson, D. J. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell 109, 693–705 (2002).
Carmeliet, P. & Tessier-Lavigne, M. Common mechanisms of nerve and blood vessel wiring. Nature 436, 193–200 (2005).
Tam, S. J. & Watts, R. J. Connecting vascular and nervous system development: angiogenesis and the blood-brain barrier. Annu. Rev. Neurosci. 33, 379–408 (2010).
Julius, D. & Basbaum, A. I. Molecular mechanisms of nociception. Nature 413, 203–210 (2001).
Caterina, M. J. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 (1997).
Patapoutian, A. & Macpherson, L. Channeling pain. Nat. Med. 12, 506–507 (2006).
Patapoutian, A., Tate, S. & Woolf, C. J. Transient receptor potential channels: targeting pain at the source. Nat. Rev. Drug Discovery 8, 55–68 (2009).
Dubin, A. E. & Patapoutian, A. Nociceptors: the sensors of the pain pathway. J. Clin. Invest. 120, 3760–3772 (2010).
Yin, C. et al. ApoE attenuates unresolvable inflammation by complex formation with activated C1q. Nat. Med. 25, 496–506 (2019).
Pavlov, V. A., Chavan, S. S. & Tracey, K. J. Molecular and functional neuroscience in immunity. Annu. Rev. Immunol. 36, 783–812 (2018).
Brunet, I. et al. Netrin-1 controls sympathetic arterial innervation. J. Clin. Invest. 124, 3230–3240 (2014).
Andersson, U. & Tracey, K. J. Reflex principles of immunological homeostasis. Ann. Rev. Immunol. 30, 313–335 (2012).
Steinman, L. Lessons learned at the intersection of immunology and neuroscience. J. Clin. Invest. 122, 1146–1148 (2012).
Dutta, P. et al. Myocardial infarction accelerates atherosclerosis. Nature 487, 325–329 (2012).
Rosas-Ballina, M. et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101 (2011).
Sudhof, T. C. et al. Synapsins: mosaics of shared and individual domains in a family of synaptic vesicle phosphoproteins. Science 245, 1474–1480 (1989).
Sudhof, T. C. Towards an understanding of synapse formation. Neuron 100, 276–293 (2018).
Zeng, W. et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 163, 84–94 (2015).
Cserep, C. et al. Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science 367, 528–537 (2020).
Stjarne, L. Basic mechanisms and local modulation of nerve impulse-induced secretion of neurotransmitters from individual sympathetic nerve varicosities. Rev. Physiol. Biochem. Pharmacol. 112, 1–137 (1989).
Magnon, C. et al. Autonomic nerve development contributes to prostate cancer progression. Science 341, 1236361 (2013).
Laine, P., Naukkarinen, A., Heikkila, L., Penttila, A. & Kovanen, P. T. Adventitial mast cells connect with sensory nerve fibers in atherosclerotic coronary arteries. Circulation 101, 1665–1669 (2000).
Chiu, I. M., von Hehn, C. A. & Woolf, C. J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 15, 1063–1067 (2012).
Han, W. et al. A neural circuit for gut-induced reward. Cell 175, 665–678 (2018).
Carnevale, D. et al. A cholinergic-sympathetic pathway primes immunity in hypertension and mediates brain-to-spleen communication. Nat. Commun. 7, 13035 (2016).
Carnevale, L. et al. Celiac vagus nerve stimulation recapitulates angiotensin II-induced splenic noradrenergic activation, driving egress of CD8 effector cells. Cell Rep 33, 108494 (2020).
Gerber, K. J., Squires, K. E. & Hepler, J. R. Roles for regulator of G protein signaling proteins in synaptic signaling and plasticity. Mol. Pharmacol. 89, 273–286 (2016).
Wirth, T. et al. The sympathetic nervous system modulates CD4+Foxp3+ regulatory T cells via noradrenaline-dependent apoptosis in a murine model of lymphoproliferative disease. Brain Behav. Immun. 38, 100–110 (2014).
Carnevale, D. et al. The angiogenic factor PlGF mediates a neuroimmune interaction in the spleen to allow the onset of hypertension. Immunity 41, 737–752 (2014).
Kaufmann, H., Norcliffe-Kaufmann, L. & Palma, J. A. Baroreflex dysfunction. New Engl. J. Med. 382, 163–178 (2020).
McMahon, S. B., La Russa, F. & Bennett, D. L. Crosstalk between the nociceptive and immune systems in host defence and disease. Nat. Rev. Neurosci. 16, 389–402 (2015).
Morrison, S. F. & Nakamura, K. Central mechanisms for thermoregulation. Annu. Rev. Physiol. 81, 285–308 (2019).
Tracey, K. J. Reflex control of immunity. Nat. Rev. Immunol. 9, 418–428 (2009).
Talbot, S., Foster, S. L. & Woolf, C. J. Neuroimmunity: physiology and pathology. Annu. Rev. Immunol. 34, 421–447 (2016).
Chu, C., Artis, D. & Chiu, I. M. Neuro-immune interactions in the tissues. Immunity 52, 464–474 (2020).
Moos, M. P. et al. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein E-deficient mice. Arter. Thromb. Vasc. Biol. 25, 2386–2391 (2005).
Lotzer, K. et al. Mouse aorta smooth muscle cells differentiate into lymphoid tissue organizer-like cells on combined tumor necrosis factor receptor-1/lymphotoxin β-receptor NF-κB signaling. Arter. Thromb. Vasc. Biol. 30, 395–402 (2010).
Kwon, J. T. et al. An amygdala circuit that suppresses social engagement. Nature 593, 114–118 (2021).
Chiang, M. C. et al. Parabrachial complex: a hub for pain and aversion. J. Neurosci. 39, 8225–8230 (2019).
Palmiter, R. D. The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci. 41, 280–293 (2018).
Han, S., Soleiman, M. T., Soden, M. E., Zweifel, L. S. & Palmiter, R. D. Elucidating an affective pain circuit that creates a threat memory. Cell 162, 363–374 (2015).
Tengeler, A. C. et al. Propionic acid and not caproic acid, attenuates nonalcoholic steatohepatitis and improves (cerebro) vascular functions in obese Ldlr−/−.Leiden mice. FASEB J. 34, 9575–9593 (2020).
Terzian, Z. et al. Peristrut microhemorrhages: a possible cause of in-stent neoatherosclerosis? Cardiovasc. Pathol. 26, 30–38 (2017).
Reeps, C. et al. Inflammatory infiltrates and neovessels are relevant sources of MMPs in abdominal aortic aneurysm wall. Pathobiology 76, 243–252 (2009).
Guzik, T. J. et al. Systemic regulation of vascular NAD(P)H oxidase activity and nox isoform expression in human arteries and veins. Arter. Thromb. Vasc. Biol. 24, 1614–1620 (2004).
Mohanta, S., Yin, C., Weber, C., Hu, D. & Habenicht, A. J. Aorta atherosclerosis lesion analysis in hyperlipidemic mice. Bio Protoc. 6, e1833 (2016).
Bianchini, M. et al. PD-L1 expression on nonclassical monocytes reveals their origin and immunoregulatory function. Sci. Immunol. 4, eaar3054 (2019).
Sun, T. et al. Tissue clearing approaches in atherosclerosis. Methods Mol. Biol. 2419, 747–763 (2022).
Cai, R. et al. Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull-meninges connections. Nat. Neurosci. 22, 317–327 (2019).
Schemann, M., Sann, H., Schaaf, C. & Mader, M. Identification of cholinergic neurons in enteric nervous system by antibodies against choline acetyltransferase. Am. J. Physiol. Gastrointest. Liver Physiol. 265, G1005–G1009 (1993).
Klupp, B. G., Granzow, H. & Mettenleiter, T. C. Primary envelopment of pseudorabies virus at the nuclear membrane requires the UL34 gene product. J. Virol. 74, 10063–10073 (2000).
Silvestre-Roig, C. et al. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature 569, 236–240 (2019).
Westphal, V. et al. Video-rate far-field optical nanoscopy dissects synaptic vesicle movement. Science 320, 246–249 (2008).
Paxinos, G. & Franklin, K. B. The Mouse Brain in Stereotaxic Coordinates (Elsevier, 2007).
Yin, C. et al. Generation of aorta transcript atlases of wild-type and apolipoprotein E-/- mice by laser capture microdissection-based mRNA expression microarrays. Methods Mol. Biol. 1339, 297–308 (2015).
Beer, M. et al. Laser-capture microdissection of hyperlipidemic/ApoE−/− mouse aorta atherosclerosis. Methods Mol. Biol. 755, 417–428 (2011).
Costantini, I. et al. A versatile clearing agent for multi-modal brain imaging. Sci. Rep. 5, 9808 (2015).
Jöns, A. & Mettenleiter, T. C. Green fluorescent protein expressed by recombinant pseudorabies virus as an in vivo marker for viral replication. J. Virol. Methods 66, 283–292 (1997).
Card, J., Whealy, M., Robbins, A., Moore, R. & Enquist, L. Two α-herpesvirus strains are transported differentially in the rodent visual system. Neuron 6, 957–969 (1991).
Cano, G., Card, J. P. & Sved, A. F. Dual viral transneuronal tracing of central autonomic circuits involved in the innervation of the two kidneys in rat. J. Comp. Neurol. 471, 462–481 (2004).
Carnevale, D. et al. Loss of EMILIN-1 enhances arteriolar myogenic tone through TGF-β (transforming growth factor-β)-dependent transactivation of EGFR (epidermal growth factor receptor) and is relevant for hypertension in mice and humans. Arter. Thromb. Vasc. Biol. 38, 2484–2497 (2018).
Carnevale, L. et al. Ultrasound-guided catheter implantation improves conscious radiotelemetric blood pressure measurement in mice. Cardiovasc. Res. 117, 661–662 (2021).
Carnevale, D. et al. Placental growth factor regulates cardiac inflammation through the tissue inhibitor of metalloproteinases-3/tumor necrosis factor-α–converting enzyme axis: Crucial role for adaptive cardiac remodeling during cardiac pressure overload. Circulation 124, 1337–1350 (2011).
Da Ros, F. et al. Targeting interleukin-1β protects from aortic aneurysms induced by disrupted transforming growth factor β signaling. Immunity 47, 959–973 (2017).
Perrotta, M. et al. Deoxycorticosterone acetate-salt hypertension activates placental growth factor in the spleen to couple sympathetic drive and immune system activation. Cardiovascular Res. 114, 456–467 (2018).
Burton, P., Gurrin, L. & Sly, P. Extending the simple linear regression model to account for correlated responses: an introduction to generalized estimating equations and multi‐level mixed modelling. Stat. Med. 17, 1261–1291 (1998).
Acknowledgements
We thank W. Wilfert for lipid analyses; V. Bontha and P. Srikakulapu for Apoe knockin breeding and aorta sections; W. L. Neuhuber for advice; M. Schemann for ChAT antibodies; M. Hong and X. Deng for technical assistance; J. Duchene for progenitor antibodies. We acknowledge the staff at Easemedcontrol for illustrations. This work was funded by The Netherlands Organisation for Applied Scientific Research (TNO): Research Program ‘Translational Pharmacology’ (KIP 10-059) and the Early Research Program ‘Body-Brain-Interactions’ (ERP 020) to R.K.; the Deutsche Forschungsgemeinschaft (DFG): MO 3054/1-1 to S.K.M., YI 133/2-1, YI 133/3-5, LMUexcellent 867949-0 to C.Y., HA 1083/15-4 and HA 1083/15-5 to A.J.R.H.; grant SFB1123-A3, DFG INST 409/209-1 FUGG and by DFG under Germany’s Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy, ID 390857198) to J.B.; Germany’s Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy, ID 390857198) to M.D.; ERA-CVD (PLAQUEFIGHT) 01KL1808 to A.J.R.H., G.L., Z. Mallat and T.J.G.; the Italian Ministry of Health (MoH): Ricerca Corrente to G.L.; the European Research Council (ERC StG 759921 SymPAtHY) to D.C.; DFG SFB 1123/Z1, INST 409/150-1 FUGG and INST409/97-1 FUGG to R.T.A.M.; the European Research Council (ERC CoG 726318 InflamaTENSION) to T.J.G.; an MRC CDA Fellowship (MR/P009824/2) to G.D.; the German Centre for Cardiovascular Research (DZHK MHA VD1.2), DFG SFB 1123/A1 and A10, the European Research Council (ERC AdG 692511) and the DFG Cluster of Excellence SyNergy (EXC 2145 SyNergy 390857198) to C.W.; C.W. is a Van de Laar professor of atherosclerosis.
Author information
Authors and Affiliations
Contributions
S.K.M. and A.J.R.H. conceived the project. S.K.M. and A.J.R.H. conceptualized the study. D.C. and G.L. conceptualized nerve recording and CGX denervation experiments. S.K.M., D.C., G.L. and A.J.R.H. designed, performed, analysed and supervised experiments, and wrote the manuscript with input from all of the authors. C.W. supervised experiments and participated in writing the manuscript. L.P. and S.K.M. designed, performed and analysed PRV tracing experiments. Y.L., S.L., T.S., L.C., M.P., Z. Ma, B.F., K.S., C.Z., X.Z., M. Bianchini, B.R.S., R.C., D.H., F.P. and L.H. performed and analysed experiments. M. Beer and D.S. participated in data analyses. P.S., R.N., T.J.G., J.P., H.-H.E., J.B.M. and Z.M. were involved in human sample analysis. B.G.K. and T.C.M. provided PRV virus constructs and contributed to design and supervision of PRV experiments. A.E., S.S., R.K. and J.B. provided materials and supervised experiments. R.T.A.M. and C.Y. performed and supervised experiments. G.D. contributed to designing and interpreting CNS neuroanatomy experiments. P.S.O. contributed to writing the manuscript. C.Y., T.C.M., R.K., J.B., T.J.G., Z. Mallat, M.D., G.D. and P.S.O. revised the manuscript. All of the authors contributed to data interpretation.
Corresponding authors
Ethics declarations
Competing interests
S.K.M., A.J.R.H. and C.Y. are owners of Easemedcontrol R&D; S.K.M., A.J.R.H., D.C., G.L., L.C., M.P., F.P. and R.C. have been named as inventors on a pending patent application (EP22153631.1, Easemedcontrol R&D) related to diagnostics and therapeutic interventions into components of the structural ABC to treat atherosclerosis.
Peer review
Peer review information
Nature thanks Ivan de Araujo, Christoph Binder, John Horn, Daniel Mucida and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Atherosclerosis-associated axon neogenesis in ATLOs.
a, Schematic view of aorta segments. b, Axon density in distinct aorta segments with or without plaque in aged WT and Apoe−/− mice; n = thorax: 361 sections in 3 WT, 121 or 209 sections in 3 Apoe−/− without or with plaque; abdomen: 254 sections in 6 WT, 109 sections in 3 Apoe−/− without plaque, 260 sections in 9 Apoe−/− with plaque. c, Axon density in thoracic (T) and abdominal (A) aorta segments with or without artery branches in non-atherosclerotic aorta segments; n = 61 or 148 sections without or with branch in 3 WT, 30 or 58 sections without or with branch in 3 Apoe−/− without plaque. d, Axon density in WT versus Apoe−/− aorta segments with plaque; n = 12–116 sections in 3 WT, 17–71 sections in 3 Apoe−/−. Abbreviations: AR, aortic root; AA, aortic arch; ICA, intercostal artery; CA, coeliac artery; SMA, superior mesenteric artery; RRA, right renal artery; LRA, left renal artery. e, Pearson correlation coefficient of axon density with plaque size in thorax and abdomen. n = 3 Apoe−/− mice. f, Enumeration of tubulin-β3 (TUBB3)+ immature axons; n = 3 WT, 3 ATLO. g, Detection of synaptophysin (Synpt+) axon endings (arrow) in ATLOs, their accumulation (arrowheads) at ATLO-media border (dashed line) and quantification in WT adventitia versus ATLOs; n = 4 WT, 4 ATLO; video 4. h, High resolution 3D reconstruction showing colocalization of CD68+ macrophages/monocytes, CD11c+ dendritic cells and CD3e+ T cells with NF200+ axons in ATLOs and their distance from adjacent axons (n = 4 ATLOs per cell-type). i, Colocalization of CD3e+ T cells with TH+ axons in ATLOs (n = 3). Arrows indicate interaction sites that are <1 μm apart; video 1. j, Synapsin (Syn)+ axon endings in WT adventitia and ATLO and accumulation of Syn+ puncta at ATLO-media border (arrowheads); video 3. n = 3 WT, 3 Apoe−/−. k,l, Colocalization of Syn+ or Synpt+ axon endings with CD45+ leukocyte forming axon-leukocyte junctions in ATLOs (arrowheads); n = 2; video 2. m, ATLOs lack ChAT+NFM+ parasympathetic axons but harbor ChAT+ leukocytes (arrow); quantification of ChAT+ T- and B- cells in ATLOs versus WT adventitia (n = 3 WT and 3 Apoe−/−) (FOV, field of view). n, Flow cytometry contour plots and quantification of ChAT+ T/B cells in ATLOs, RLNs and spleen (Spl) (n = 3 independent experiments, 2-3 mice per experiment). o-r, ADRβ2 expression in ATLOs. o, Differential gene expression of Adrb subtypes in aged WT versus Apoe−/− adventitia or plaque (n = 6 WT, 4 Apoe−/− no plaque, 4 ATLO, 3 plaque). p, 3D surface rendering of ADRβ2 expression in CD3e+ T cell in ATLO (n = 3). q, Flow cytometry gating strategy for immune cells. r, Flow cytometry contour plots and quantification of ADRβ2-expressing CD4+ T cells, CD4+CD44+CD62L- TEM cells, CD8+ T cells and B220+ B cells in ATLOs versus RLNs and spleen (n = 5 independent experiments, 1-2 mice per experiment). DAPI stains DNA in blue. For g,j, scale bars, 5µm; for h,i,k,l,p, scale bars, 2 µm; for m, scale bar, 50 µm. For b-d, center lines are medians, box limits are upper and lower quartiles and whiskers are 1.5x interquartile range. For f-h,m-o,r, data are means ± s.e.m. n represents biologically independent animals. Generalized estimating equations (b,c,d); two-sided unpaired Student´s t-test (f,g,m); one-way ANOVA with Bonferroni post hoc test (h,n,r); Generalized linear model with Bonferroni post hoc test (o)
Extended Data Fig. 2 Aorta transcriptomes for atherosclerosis-associated axon neogenesis in ATLOs.
a-d, Gene expression in WT and Apoe−/− aorta during ageing. a, Heatmaps of differentially regulated NS development genes (left) and respective NS-related GO terms (right). b-d, Quantification of selected candidate genes for axon neogenesis (b), axon guidance (c), and synaptic transmission (d). n = 3 WT, 3 Apoe−/− aorta per group. e-h, Gene expression in aged aorta laminae. e, Heatmaps show differentially expressed NS-related GO terms in laser capture microdissection-derived aged WT vs Apoe−/− aorta adventitia and plaque. f-h, Quantification of candidate genes associated with axon neogenesis (f), axon guidance (g), and synaptic transmission (h). n = 3 WT adventitia, 4 Apoe−/− adventitia no plaque, 4 ATLO, 3 plaque. i, Schematic view of laser capture microdissection-derived aged aorta laminae, and numbers of statistically significant (t-test with Benjamini-Hochberg correction) and differentially regulated NS development genes in two-tissue comparisons. n = 3 WT, 4 Apoe−/− no plaque, 4 ATLO, 3 plaque. j, Heatmaps show differentially expressed NS-related GO terms in aged WT and Apoe−/− RLN vs ATLO. k, Quantification of selected candidate genes. l,m, Comparison of selected candidate NS-genes in aged WT vs Apoe−/− RLN (l) and spleen (m). n = 3 WT RLN or spleen, 3 Apoe−/− RLN or spleen, and 4 ATLOs. Cluster analyses were performed using ANOVA with Benjamini-Hochberg correction (a,e,j). Signal intensities and statistics are reported in supplementary Tables 1–5. n, Detection and enumeration of ALDH1+ axons (arrow) in WT adventitia vs ATLO (n 3WT, 5 Apoe−/−). Scale bar, 20 µm. o, Detection of NGF-expressing periaxonal cells (arrow) and non-neuronal cells (arrowhead), and their quantification in WT adventitita versus ATLOs (n = 3 WT, 3 Apoe−/−). Scale bar, 20 µm. Inset: higher magnification of the boxed area. Scale bar, 8 µm. Data represent means ± s.e.m. n represents biologically independent animals. Generalized linear model with Bonferroni post hoc test (b-d,f-h,k); two-sided unpaired Student´s t-test (n,o); multiple unpaired t-test corrected for multiple comparisons (Bonferroni) (l,m)
Extended Data Fig. 3 Axon neogenesis, relation to genotype, age, and cause-effect relationship in adventitia NICIs.
a, NF200+ axon density in subclavian and renal arteries in aged (78 weeks) WT vs Apoe−/− mice with plaque. n = 3 WT, 3 Apoe−/−. b, Similar NF200+ axon density in renal LNs and in splenic red pulp (RP) or white pulp (WP) of aged WT vs Apoe−/−. n = RLN: 3 WT and 3 Apoe−/−; spleen: 5 WT and 4 Apoe−/−. c, Analyses of serum and spleen noradrenaline levels during ageing. n = serum: young: 3 WT, 8 Apoe−/−; adult: 6 WT, 5 Apoe−/−; aged: 7 WT, 7 Apoe−/−; spleen: young: 3 WT, 9 Apoe−/−; adult: 9 WT, 9 Apoe−/−; aged: 8 WT, 12 Apoe−/−. d, NF200+, TH+, and CGRP+ axon density in the aortic root adventitia adjacent to plaques (paraffin sections) in adult (30 weeks) Ldlr−/− on a Western diet (WD) (n = 5) vs WT mice (n = 4). e, NF200+, TH+, and CGRP+ axon density in the aortic root adventitia (frozen sections) in adult (32 weeks) Apoe−/− (n = 4) versus WT mice (n = 3) . f, Analysis of aortic arch noradrenaline levels in adult mice. n = 3 WT, 4 Apoe−/−. g, Representative OR/H image of abdominal aorta of aged humanized Apoe4 knockin mice on high-fat diet (HFD); comparison of serum total cholesterol (n = 16 WT, 21 Apoe−/−, 8 Apoe4 on chow diet, 10 Apoe4 HFD); and NF200+, TH+, and CGRP+ axon densities in the abdominal aorta adventitia of Apoe4 HFD vs Apoe4 mice (n = 3 Apoe4, 4 Apoe4 HFD). Scale bar, 50 µm. h, Representative OR/H image of abdominal aorta of aged Apoe−/−/Ltbrfl/fl/Tagln-cre mice, and axon densities in the abdominal adventitia of aged Apoe−/−/Ltbrfl/fl/Tagln-cre compared to their Apoe−/− control or WT adventitia. n = 6 WT, 6 Apoe−/−, 4 Apoe−/−/Ltbrfl/fl/Tagln-cre. Scale bar, 25 µm. Data are means ± s.e.m. n represents biologically independent animals. Two-way ANOVA with Bonferroni post hoc test (a,b,c,d,e,g); two-sided unpaired Student´s t-test (f); factorial ANOVA with Bonferroni post hoc test (h)
Extended Data Fig. 4 Restructuring of the PNS in the vicinity of adventitia NICIs.
a, Approach on tracing in tissue-cleared intact abdominal aorta of young (12 weeks) WT mice. 3D segmentation and manual tracing of NF200+ nerves/axon bundles along their entire paths (800–1200 µm thickness) shows connectivities of the sympathetic CG (yellow) and SycGs (light yellow) with the adventitia. An example of a traced axon bundles (red) contacting the adventitia; approximations of diameters of different sizes of nerves/axon bundle connecting the ganglia are indicated. Arrowheads, beginning and ending of the traced axon bundle from adventitia to CG; arrow, direction of tracing. Scale bars, 0.7 mm. b, Light sheet 3D reconstruction images of NF200+ nerves and axons in the intact abdominal aorta and periaortic tissues (autofluorescence, magenta) of aged WT (n = 3) and Apoe−/− (n = 2) mice showing NF200+ neuronal structures (green) in the 600 µm-thick abdominal aortic segments (4 µm per z-step; arrow, CG; arrowhead, SycGs). Scale bars, 0.5 mm. c, Light sheet 3D reconstruction in the intact abdominal aorta of aged Apoe−/− mice showing varicosities (arrows) in axons adjacent to plaque. Inset, single plan image of the 3D projected whole stack (z = 1.2 mm; arrow, varicosities in axons; arrowhead, nerve) (c). Dashed line, media; yellow line, plaque. Scale bars, 500 µm (left), 50 µm (rihgt). d,e, 3D reconstruction and segmentation views of an abdominal aorta region showing NF200+ nerves and axons and their spatial relationship to the intact abdominal aorta (z = 320 µm; 4 µm per z-step) in aged Apoe−/− mice (n = 2). Nerves and axons are evident in the vicinity of the aorta; video 11. Scale bars, 40 µm. f, 3D reconstruction of TDE-cleared whole mount abdominal aorta (z = 80 µm; 1 µm per z-step; n = 3) showing NF200+ nerves and axons in the adventitia. White, second harmonic generation (SHG) from collagen in the adventitia. Arrow, axon; double arrowhead, nerve; asterisk, axon neogenesis in ATLO. Scale bars, 70 µm. g, NF200+ axons (arrow, arrowhead) in T cell (T) and B cell (B) areas in ATLO, and their quantification in ATLO and Apoe−/− paraaortic lymph node (pLN). n = 7 ATLOs, 4 pLNs. Scale bar, 30 µm. h, Single neurofilament L (NFL)+ immature newly formed axons and double NFL+/neurofilament H (NFH)+ mature axons in aged WT adventitia vs ATLOs (n = 3 WT, 3 Apoe−/−). Scale bars, 10 µm. Data are means ± s.e.m. n represents biologically independent animals. Two-sided unpaired Student´s t-test (g); two-way ANOVA with Bonferroni post hoc test (h)
Extended Data Fig. 5 NICIs in human cardiovascular tissues.
a,b, Representative images and quantification of NF200+ and TH+ axons in the coronary artery (CA) adventitia of healthy control donors vs atherosclerotic CA of cardiac transplant recipients with coronary artery disease (CAD) (paraffin sections, n = 5 healthy CA from organ donors, 10 CA (NF200) or 6 CA (TH) with plaque). Representative images and quantification of NF200+ and TH+ axons in non-atherosclerotic CAs versus early atherosclerotic CA adventitia from cardiac transplant recipients (frozen sections, n = 6 CA (NF200) or 4 CA (TH) nonCAD without plaque; 7 CA (NF200) or 5 CA (TH) CAD with plaque). c, Detection and enumeration of NF200+ and TH+ nerves in the abdominal aorta adventitia of healthy control donors, non-atherosclerotic and atherosclerotic asymptomatic abdominal aortic aneurysm (AAA) aorta (paraffin sections); n = 4 healthy abdominal aorta, 5 AAA no plaque, 16 AAA with plaque. d, Aortic noradrenaline levels in healthy vs and atherosclerotic AAA aorta; n = 5 healthy abdominal aorta, 8 AAA with plaque. e, Detection and quantification of NF200+ and TH+ axons in AAA with TLOs versus without TLOs (frozen sections); n = 5 AAA no TLO, 6 AAA with TLO. f, H&E-stained nerve-TLCs (N-TLCs) in healthy versus atherosclerotic CAs; n = 5 healthy CA from organ donors, 10 CA with plaque. g, CD45+ N-TLCs in adventitial nerves in healthy versus atherosclerotic AAA aorta; n = 7 healthy control aorta, 13 AAA with plaque. h, CD45+ leukocyte infiltration in adventitial nerves in healthy versus atherosclerotic AAA aorta. n = 4 healthy control aorta, 10 AAA with plaque. For a,b,e, scale bars, 25 µm; for c,f-h, scale bars, 100 µm. Data are means ± s.e.m. n represents biologically independent samples. Two-sided unpaired Student´s t-test (a,b,d,e); one-way ANOVA with Bonferroni post hoc test (c); two-sided Mann-Whitney U-test (f-h). Patient details are reported in supplementary Tables 7 and 8
Extended Data Fig. 6 NICIs in PNS ganglia and nerves in atherosclerosis.
a, OR/H-staining shows the presence of epineural tertiary lymphoid cluster (TLC) surrounding PvaGs in aged Apoe−/− mice, but not in aged WT mice (dashed line, media) (n = 12 WT, 20 Apoe−/−). b-f, Cellularity and structures of TLCs. b, TH+ sympathetic PvaG-TLCs harbor CD45+ leukocytes including CD68+ macrophages (arrow), CD11c+ MHC-II+ dendritic cells (arrow), CD3e+ T cells (open triangle), B220+ B cells (filled triangle), CD138+ plasma cells (arrow). c, Foxp3+ T regulatory cells (arrow), Ki67+ proliferating centrocytes in germinal centre (PNA+) (filled triangle), Ki67+ proliferating B cells (arrow), IgM+ plasma cells (filled triangle). d, PNAd+ high endothelial venules (HEV) (arrow), Coll-IV+ or Meca32+ blood vessels (open arrow, arrow), Lyve1+ lymph vessels (open triangle), ER-TR7+ conduits (open arrow) or ER-TR7+ epineurium (filled triangle) and their connection with HEVs (arrow). e, Nerve-TLCs contain CD45+ leukocytes including CD68+ macrophages, CD3e+ T cells (open triangle), B220+ B cells (filled triangle), CD138+ plasma cells (arrow), PNAd+ HEVs (arrow), Meca 32+ blood vessels (arrow), Lyve1+ lymph vessels (open triangle), and ER-TR7+ conduits (open arrow). f, DRG-TLCs around epineuria adjacent to spinal meninges (arrow head) contain CD68+ macrophages (arrow) and B220+ B cells (f). n = PvaG: 19 Apoe−/−; nerves: 12 Apoe−/−; DRGs: 7 Apoe−/−. g, Morphometry of epineural clusters in PvaG (n = 12 WT, 20 Apoe−/−), nerves (n = 8 WT, 12 Apoe−/−) and DRGs (n = 5 WT, 7 Apoe−/−) in aged mice. Each sphere represents the total number of clusters per mouse. h, Pearson correlation coefficient of PvaG-TLC sizes (TLC/PvaG area) with both plaque sizes (intima/media area) and ATLO sizes (adventitia/media area) (n = 15 PvaG-TLCs). One symbol represents the mean value of one individual variable. i, TLO stages of epineural clusters in PvaG (n = 19 Apoe−/−), nerves (n = 12 Apoe−/−) and DRGs (n = 7 Apoe−/−). Each sphere represents TLO stages per tissue. j, Heatmaps of LCM-derived PvaG microarrays show differentially regulated genes in respective immuno-inflammation-related GO terms in aged WT versus Apoe−/− PvaGs. Analyses were performed using two-sided unpaired Student’s t test. n = 5 WT PvaGs, 6 Apoe−/− PvaGs. k,l, Quantitative comparisons of differentially expressed up-regulated genes for cytokine activity, mast cell activation, complement activation, nervous system development, and axonogenesis in WT versus Apoe−/− PvaGs. (n = 5 WT PvaGs, 6 Apoe−/− PvaG). Signal intensities and statistics are reported in supplementary Table 6. m, Sympathetic gene expression in LCM-derived PvaG. n = 8 PvaGs. p, Detection of CXCL13 expression in WT and Apoe−/− PvaGs in B cell follicles (open triangle) and in PvaG neuronal cell bodies (filled triangle). o, Schematic choreographies of PvaG-TLCs, N-TLCs, and DRG-TLCs. n, Enumeration of infiltrating intraganglionic CD68+ macrophages, CD3e+ T cells, and Giemsa-stained mast cells within PvaGs, and thoraco-lumbar DRGs (i). n = PvaG: 6 WT, 8 Apoe−/−; DRGs: 3 WT, 5 Apoe−/−. Scale bars, 50 µm. Data are means ± s.e.m. n represents biologically independent animals. Two-sided Mann-Whitney U-test (g); Pearson bivariate correlation (h); Kruskal-Wallis H test (i); multiple unpaired t-test corrected for multiple comparisons (Bonferroni) (k,l); one way ANOVA with Bonferroni post hoc test (m); Two-sided unpaired Student´s t-test (n)
Extended Data Fig. 7 Distinct pattern of CNS nuclei participates in the formation of the ABC.
a, Schematic of PRV injection site in the perirenal abdominal aorta. b, Schematic of polysynaptic PRV retrograde migration from ATLO to brain. c, In-situ detection of India ink around the abdominal aorta and in RLN (left), but not in the kidney (right). Histological detection of ink in the adventitia, paraaortic adipose tissue (arrow) and within RLN (arrowhead), but not in the PvaG soma 2 days post-injection (n = 5 aged WT). d, PRV+ neurons (arrow) in TH+ sympathetic PvaGs at d2 or SycGs at d4 p.i., and their quantification. e, PRV+ neurons in thoracic 6–13th DRGs of aged Apoe−/− mice at d4; n = 4 per time point. f, Mapping of PRV+ neurons from ATLO to brain until d4. n = 4 for PvaG, 3 for CG; 5 for IML; 6 for RPa; 6 for PVN . Each circle represents mean of PRV+ neurons, arrow indicates direction of PRV migration, and colour indicates the p.i. day. g, Quantification PRV+ neurons in 27 distinct brain nuclei at 4–6d after abdominal aorta inoculation; n = 6 per time point. h, Anatomical locations of brain nuclei in g according to the Allen Mouse Brain Atlas (sagittal view). i, Connectivity mapping of PRV migration over time in 28 different neural tissues (from peripheral ganglia to higher brain nuclei depicted in f,g) after abdominal aorta inoculation. j, Anatomical representation (sagittal view) of neural tissues in i according to the Allen Mouse Brain Connectivity Atlas. Each circle represents mean of PRV+ neurons, arrow indicates direction of PRV migration, and colour indicates the p.i. day. k,l, Quantitative comparisons of PRV+ neurons in IML of the spinal cord and 27 distinct brain nuclei (k), and their anatomical mapping (l) at 6d p.i. into the abdominal aorta versus control target tissues: right eye, psoas major lumbar muscle, kidney, and blood; n = 4 per group. Scale bars, 50 µm. Data are means ± s.e.m. n represents biologically independent animals. Mixed-model ANOVA with Bonferroni post hoc test (d,e). Abbreviations: gigantocellular reticular nucleus-alpha (GiA), raphe pallidus (RPa), lateral paragigangtocellular nuclei (LPGi), lateral reticular nucleus (LRt); Raphe obscurus nucleus (Rob); rostroventral lateral medulla (RVLM); area postrema (AP); repositus nucleus (Pr); locus coeruleus (LC); Barrington’s nucleus (Bar); noradrenaline cell group 5 (A5); laterodorsal tegmental nucleus (LDTg); ventrolateral periaqueductal gray (VLPAG); lateral periaqueductal gray (LPAG); medial lemniscus (ml); peduncular part of lateral hypothalamus (PLH); dorsomedial hypothalamic nucleus (DM); ventromedial hypothalamic nucleus (VMH); arcuate hypothalamic nucleus (Arc); suprachiasmatic nucleus (Sch); cortical amygdala (CoA); lateral amygdala (LA); medial amygdala (MeA); central amygdala (CeA); intergeniculate leaflet of the thalamus (IGL); and piriform cortex (Pir)
Extended Data Fig. 8 Specificity of ABC circuit in aged Apoe−/− mice.
a,b, ATLOs are innervated by nodose ganglia (NG) neurons or other parasympathetic efferents. a, Localization and quantification of PRV+ neurons in the NG, 10N and NTS in 3–6d p.i. after abdominal aorta inoculation. Detection of PRV+ChAT+ cholinergic neurons in 10N at d6 after abdominal aorta inoculation (right). n = d4: 3 mice; d5: 3 mice (NG,10N); 6 mice (NTS); d6: 4 mice (NG); 6 mice (10N, NTS). Arrow indicates double positive neuron. b, Localization and quantification of PRV+ neurons in the NG, 10N and NTS in 2–4d p.i. after stomach wall inoculation; n = d2,3: 3 mice; d4: 4 mice. c, Quantification of PRV+ neurons in medulla, hypothalamic and amygdala nuclei at d6 p.i. in the abdominal aorta; n = 3 WT, 4 Apoe−/−. d, PRV+TH+ neurons among total TH+ neurons in sympathetic brain nuclei including RVLM, LC and A5 in aged WT versus Apoe−/− mice; n = 3 WT, 3 Apoe−/−. e, Quantification of Fos+NeuN+ neurons in CoA, LA, MeA nuclei of the amygdala); n = 3 WT, 3 Apoe−/−. f, Detection of Fos+NeuN+ and Fos+TH+ neurons in RPa (arrow); n = 2 WT, 3 Apoe−/−. g, Detection of Fos+ChAT+ neurons in LPGi (arrow). n = 2 WT, 2 Apoe−/−. h, Detection and quantification of Fos+NeuN+ neurons among total NeuN+ neurons in TH+ LC (arrow); n = 3 WT, 4 Apoe−/−. i, Detection of Fos+ neurons within CGRP+ axon field in the NTS. n = 2 WT, 2 Apoe−/−. Scale bars, 50 µm. Data are means ± s.e.m. n represents biologically independent animals. Mixed-model ANOVA with Bonferroni post hoc test (a,b); multiple unpaired t-test corrected for multiple comparisons (Bonferroni) (c,d,e); two-sided unpaired Student´s t-test (h)
Extended Data Fig. 9 Atherosclerosis, cardiac imaging, blood pressure and heart rate variability.
a, In vivo ultrasound images followed by 3D reconstructions of aorta and plaque volumes versus corresponding in-situ images in aortic arch and abdominal aorta of WT and Apoe−/− mice (n = young: 4 WT, 8 Apoe−/−; adult: 12 WT, 17 Apoe−/−; aged: 21 WT, 24 Apoe−/−); video 13. b-e, Imaging of an abdominal plaque using ultrasound-imaging and histology in aged Apoe−/− mice. b, B-mode echo images of the abdominal plaque (P) (yellow line). c, 3D reconstruction of B-mode echo images (plaque in yellow). d, Histological detection of OR/H stained lipid-rich plaque area (red). e, Pearson correlation of abdominal aorta plaque volume measured by ultrasound versus plaque size (intima/media ratio) measured by histology. n = 9 aged Apoe−/− mice for ultrasound imaging before sacrifice and for histology in the same mice postmortem. Abbreviations: RRA, right renal artery; Abd. aorta, abdominal aorta. f, Echocardiographic assessment of cardiac functional and structural parameters in aged WT and Apoe−/− mice (n = 20 WT and 20 Apoe−/−). g, Analysis of the heart rate variability (HRV) across life span in young and aged Apoe−/− vs WT mice. (n = young: 4 WT, 4 Apoe−/−; aged: 3 WT, 4 Apoe−/−). h, Radiotelemetric analysis of systolic and diastolic blood pressure measurements in young and aged Apoe−/− vs WT mice across lifespan (n = young: 4 WT, 4 Apoe−/−; aged: 3 WT, 4 Apoe−/−). Blood pressure and HRV were continuously measured for 3 days, during night and day. Average values of nocturnal and diurnal blood pressure for the 3 days of measurements are shown. Measurements of cardiovascular parameters during ageing are reported in supplementary Tables 9 and 10. For a,b,f, scale bars, 1 cm; for d, scale bar, 50 µm. For f, centre lines are medians, box limits are upper and lower quartiles and whiskers are 1.5x interquartile range. For g-h, data are means ± s.e.m. n represents biologically independent animals. Two-sided unpaired Student´s t-test (f); mixed-model ANOVA with Bonferroni post hoc test (g,h)
Extended Data Fig. 10 The SNS promotes atherosclerosis during ageing.
a, Spearman correlation coefficients of SSNA vs plaque volume in aortic arch and abdominal aorta in young, adult and aged Apoe−/− mice (n = 26 mice). b, Representative raw signals of SSNA in a time window of 10 min and relative quantification of SSNA spikes in adult and aged Apoe−/− mice before and after Coeliac vagotomy (CVNX) (n = 6 adult and 7 aged). c, Levels of neuron activation-related genes in LCM-derived sympathetic PvaGs in aged WT versus Apoe−/− mice (n = 5 WT PvaGs, 6 Apoe−/− PVaGs). Signal intensities and statistics are reported in supplementary Table 6. d, Approach to 4 weeks 6-OHDA-induced chemical sympathetic denervation in aged Apoe−/− mice. e, TH+ neurons in the locus coeruleus (n = 5 control and 5 OHDA). f, Analysis of spleen noradrenaline (n = 4 control and 7 OHDA), splenic TH+ area (n = 4 control and 4 OHDA), and aortic root TH+ area (n = 3 control and 3 OHDA). g,h, Effect of 6-OHDA denervation on plaque and ATLO. g, OR/H stained abdominal aorta showing ATLO cellularity. (h, Quantification of abdominal aorta media area; macrophage area (CD68+), necrotic core area, SMC area (SMA+), collagen area (Sirius red+), fibrous cap thickness in plaque; and CD3e+Foxp3+ T regulatory cells in ATLO ; n = 4 control and 4 OHDA. i, Measurement of serum cholesterol (n = 6 control and 8 OHDA), relative organ weight (n = 5 per group). j, Flow cytometry gating strategies and enumeration of Lin-Sca1+Kit+CD150+CD48- haematopoietic stem cells (HSC) gated from LSK (Lin-Sca1+Kit+) or Lin-Sca1-Kit+CD34+CD16/32+ granulocyte-macrophage progenitors (GMP) gated from myeloid progenitor cells (MPC) (Lin-Sca1+Kit-) in the bone marrow from total live cells (n = 5 control and 5 OHDA). (k, Gating strategies and quantification of CD11c+CD11b+ myeloid cells and CD4+Foxp3+ T regulatory cells in spleen and RLN from total live cells (n = 5 control and 5 OHDA). l, Approach to 8 months CGX selective surgical denervation in adult Apoe−/− mice. m, Analysis of spleen noradrenaline (n = 4 sham and 5 CGX), aortic root TH+ area (n = 4 sham and 5 CGX), and root plaque size (n = 4 sham and 7 CGX). n, Changes in serum cholesterol (n = 3 sham and 9 CGX); relative organ weights after 8 months of surgery (n = 4 sham and 9 CGX). o, Flow cytometry gating strategy and quantification of number of CD11b+ myeloid cells and CD4+Foxp3+ T regulatory cells in spleen and RLN from total live cells (n = 4 sham; 4 CGX). p,q, Effect of surgical denervation on ATLO and plaque cellularity. p, Histological staining shows ATLO cellularity., q, Quantification of abdominal aorta media area; macrophage area (CD68+), necrotic core area, SMC area (SMA+), collagen area (Sirius red+), fibrous cap thickness in plaque; and CD3e+Foxp3+ T regulatory cells in ATLO ; n = 4 sham, 5 CGX. r, Analyses of internal diameter and ß-stiffness in ascending aorta and abdominal aorta of sham and CGX- mice before surgery (Basal) and 8 months after surgery. s, Systolic and diastolic blood pressure measurement (Basal) and at every 2 months up to 8 months after surgery n = r,s: 7 sham, 10 CGX before surgery, and 5 sham, 9 CGX at 8 months post-CGX.For e, scale bars, 20 µm; for g,p, scale bars 50 µm. Data are means ± s.e.m. n represents biologically independent animals. Two-way ANOVA with Bonferroni post hoc test (b,j,k,o); two-sided unpaired Student´s t-test (e,f,h,i,m,n,q); multiple unpaired t-test corrected for multiple comparisons (Bonferroni) (c,i,n); mixed-model ANOVA with Bonferroni post hoc test (r,s). t, Comparison of short-term (4 weeks) pharmacological deletion of the SNS and long-term (8 months) outcome of CGX on plaque and ATLO
Extended Data Fig. 11 Schematics of ABC sensor and ABC effector.
a, Adventitia NICIs initiate the ABC using sensory neurons of DRGs to enter the CNS via the spinal cord dorsal horn and—from there—projects to the brain stem medulla oblongata. b, SNS efferents project from hypothalamic and brainstem nuclei to the spinal cord and—from there—to the adventitia via the CG, while vagal efferents originating in the medulla oblongata project to the CG—after traversing the NG in the neck—to create an ABC effector.
Supplementary information
Supplementary Information
Legends for Supplementary Tables 1–10 and legends for Supplementary Videos 1–13.
Source data
Rights and permissions
About this article
Cite this article
Mohanta, S.K., Peng, L., Li, Y. et al. Neuroimmune cardiovascular interfaces control atherosclerosis. Nature 605, 152–159 (2022). https://doi.org/10.1038/s41586-022-04673-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-04673-6
This article is cited by
-
The effect of carotid sinus neurectomy for carotid restenosis: a study protocol for a double-blinded and randomized controlled trial
Trials (2024)
-
Circulating mitochondria promoted endothelial cGAS-derived neuroinflammation in subfornical organ to aggravate sympathetic overdrive in heart failure mice
Journal of Neuroinflammation (2024)
-
Immune and inflammatory mechanisms in hypertension
Nature Reviews Cardiology (2024)
-
Somatosensory cortex and central amygdala regulate neuropathic pain-mediated peripheral immune response via vagal projections to the spleen
Nature Neuroscience (2024)
-
Canonical and non-canonical roles of complement in atherosclerosis
Nature Reviews Cardiology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.