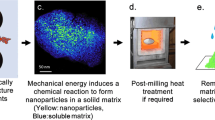
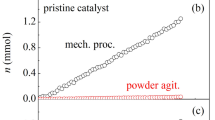
Abstract
Owing to its efficiency and unique reactivity, mechanochemical processing of bulk solids has developed into a powerful tool for the synthesis and transformation of various classes of materials. Nevertheless, mechanochemistry is primarily based on simple techniques, such as milling in comminution devices. Recently, mechanochemical reactivity has started being combined with other energy sources commonly used in solution-based chemistry. Milling under controlled temperature, light irradiation, sound agitation or electrical impulses in newly developed experimental setups has led to reactions not achievable by conventional mechanochemical processing. This Perspective describes these unique reactivities and the advances in equipment tailored to synthetic mechanochemistry. These techniques — thermo-mechanochemistry, sono-mechanochemistry, electro-mechanochemistry and photo-mechanochemistry — represent a notable advance in modern mechanochemistry and herald a new level of solid-state reactivity: mechanochemistry 2.0.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
James, S. L. et al. Mechanochemistry: opportunities for new and cleaner synthesis. Chem. Soc. Rev. 41, 413–447 (2012).
Do, J.-L. & Friščić, T. Mechanochemistry: a force of synthesis. ACS Cent. Sci. 3, 13–19 (2017).
Friščić, T., Mottillo, C. & Titi, H. M. Mechanochemistry for synthesis. Angew. Chem. Int. Ed. 59, 1018–1029 (2020).
Michalchuk, A. A. L., Boldyreva, E. V., Belenguer, A. M., Emmerling, F. & Boldyrev, V. V. Tribochemistry, mechanical alloying, mechanochemistry: what is in a name? Front. Chem. 9, 685789 (2021).
Boldyrev, V. V. & Tkáčová, K. Mechanochemistry of solids: past, present, and prospects. J. Mater. Synth. Process. 8, 121–132 (2000).
Braga, D., Maini, L. & Grepioni, F. Mechanochemical preparation of co-crystals. Chem. Soc. Rev. 42, 7638–7648 (2013).
Solares-Briones, M. et al. Mechanochemistry: a green approach in the preparation of pharmaceutical cocrystals. Pharmaceutics 13, 790–839 (2021).
Wang, G.-W. Mechanochemical organic synthesis. Chem. Soc. Rev. 42, 7668–7700 (2013).
Andersen, J. & Mack, J. Mechanochemistry and organic synthesis: from mystical to practical. Green Chem. 20, 1435–1443 (2018).
Friščić, T. in Ball Milling Towards Green Synthesis: Applications, Projects, Challenges (eds Stolle, A. & Ranu, B.) 151–189 (Royal Society of Chemistry, 2015).
Stolar, T. & Užarević, K. Mechanochemistry: an efficient and versatile toolbox for synthesis, transformation, and functionalization of porous metal–organic frameworks. CrystEngComm 22, 4511–4525 (2020).
Peh, S. B., Wang, Y. & Zhao, D. Scalable and sustainable synthesis of advanced porous materials. ACS Sustain. Chem. Eng. 7, 3647–3670 (2019).
Bennett, T. D. et al. Facile mechanosynthesis of amorphous zeolitic imidazolate frameworks. J. Am. Chem. Soc. 133, 14546–14549 (2011).
Stolar, T. et al. Scalable mechanochemical amorphization of bimetallic Cu–Zn MOF-74 catalyst for selective CO2 reduction reaction to methanol. ACS Appl. Mater. Interfaces 13, 3070–3077 (2021).
Willart, J. F. & Descamps, M. Solid state amorphization of pharmaceuticals. Mol. Pharm. 5, 905–920 (2008).
Boldyreva, E. Mechanochemistry of inorganic and organic systems: what is similar, what is different? Chem. Soc. Rev. 42, 7719–7738 (2013).
Takacs, L. The historical development of mechanochemistry. Chem. Soc. Rev. 42, 7649–7659 (2013).
Ardila‐Fierro, K. J. & Hernández, J. G. Sustainability assessment of mechanochemistry by using the twelve principles of green chemistry. ChemSusChem 14, 2145–2162 (2021).
Colacino, E., Delogu, F. & Hanusa, T. Advances in mechanochemistry. ACS Sustain. Chem. Eng. 9, 10662–10663 (2021).
Howard, J., Cao, Q. & Browne, D. L. Mechanochemistry as an emerging tool for molecular synthesis: what can it offer? Chem. Sci. 9, 3080–3094 (2018).
Braga, D. et al. Solvent effect in a “solvent free” reaction. CrystEngComm 9, 879–881 (2007).
Martinez, V. et al. Tunable fulleretic sodalite MOFs: highly efficient and controllable entrapment of C60 fullerene via mechanochemistry. Chem. Mater. 32, 10628–10640 (2020).
Pickhardt, W., Grätz, S. & Borchardt, L. Direct mechanocatalysis: using milling balls as catalysts. Chemistry 26, 12903–12911 (2020).
Lukin, S. et al. Mechanochemical carbon–carbon bond formation that proceeds via a cocrystal intermediate. Chem. Commun. 54, 13216–13219 (2018).
Štrukil, V., Gracin, D., Magdysyuk, O. V., Dinnebier, R. E. & Friščić, T. Trapping reactive intermediates by mechanochemistry: elusive aryl N-thiocarbamoylbenzotriazoles as bench-stable reagents. Angew. Chem. Int. Ed. 54, 8440–8443 (2015).
Katsenis, A. D. et al. In situ X-ray diffraction monitoring of a mechanochemical reaction reveals a unique topology metal-organic framework. Nat. Commun. 6, 6662 (2015).
Ayoub, G. et al. Rational synthesis of mixed-metal microporous metal–organic frameworks with controlled composition using mechanochemistry. Chem. Mater. 31, 5494–5501 (2019).
Bolm, C. & Hernández, J. G. Mechanochemistry of gaseous reactants. Angew. Chem. Int. Ed. 58, 3285–3299 (2019).
Eckert, R., Felderhoff, M. & Schüth, F. Preferential carbon monoxide oxidation over copper-based catalysts under in situ ball milling. Angew. Chem. Int. Ed. 56, 2445–2448 (2017).
Do, J.-L. & Friščić, T. Chemistry 2.0: developing a new, solvent-free system of chemical synthesis based on mechanochemistry. Synlett 28, 2066–2092 (2017).
Gomollón-Bel, F. Ten chemical innovations that will change our world: IUPAC identifies emerging technologies in chemistry with potential to make our planet more sustainable. Chem. Int. 41, 12–17 (2019).
Friščić, T., Trask, A. V., Jones, W. & Motherwell, W. D. S. Screening for inclusion compounds and systematic construction of three-component solids by liquid-assisted grinding. Angew. Chem. Int. Ed. 45, 7546–7550 (2006).
Shan, N., Toda, F. & Jones, W. Mechanochemistry and co-crystal formation: effect of solvent on reaction kinetics. Chem. Commun. 2, 2372–2373 (2002).
Friščić, T., Childs, S. L., Rizvi, S. A. A. & Jones, W. The role of solvent in mechanochemical and sonochemical cocrystal formation: a solubility-based approach for predicting cocrystallisation outcome. CrystEngComm 11, 418–426 (2008).
Cao, Q., Howard, J. L., Crawford, D. E., James, S. L. & Browne, D. L. Translating solid state organic synthesis from a mixer mill to a continuous twin screw extruder. Green Chem. 20, 4443–4447 (2018).
Stolar, T. et al. Control of pharmaceutical cocrystal polymorphism on various scales by mechanochemistry: transfer from the laboratory batch to the large-scale extrusion processing. ACS Sustain. Chem. Eng. 7, 7102–7110 (2019).
Hernández, J. G. C–H bond functionalization by mechanochemistry. Chemistry 23, 17157–17165 (2017).
Bowmaker, G. A. Solvent-assisted mechanochemistry. Chem. Commun. 49, 334–348 (2013).
Lapshin, O. V., Boldyreva, E. V. & Boldyrev, V. V. Role of mixing and milling in mechanochemical synthesis (Review). Russ. J. Inorg. Chem. 66, 433–453 (2021).
Delogu, F. & Takacs, L. Information on the mechanism of mechanochemical reaction from detailed studies of the reaction kinetics. J. Mater. Sci. 53, 13331–13342 (2018).
Horie, K. et al. Definitions of terms relating to reactions of polymers and to functional polymeric materials (IUPAC Recommendations 2003). Pure Appl. Chem. 76, 889–906 (2004).
Baláž, P. Mechanochemistry in Nanoscience and Minerals Engineering Ch. 1 (Springer, 2008).
Haley, R. A., Mack, J. & Guan, H. 2-in-1: catalyst and reaction medium. Inorg. Chem. Front. 4, 52–55 (2017).
Haley, R. A., Zellner, A. R., Krause, J. A., Guan, H. & Mack, J. Nickel catalysis in a high speed ball mill: a recyclable mechanochemical method for producing substituted cyclooctatetraene compounds. ACS Sustain. Chem. Eng. 4, 2464–2469 (2016).
Tireli, M. et al. Solvent-free copper-catalyzed click chemistry for the synthesis of N-heterocyclic hybrids based on quinoline and 1,2,3-triazole. Beilstein J. Org. Chem. 13, 2352–2363 (2017).
Vogt, C. G. et al. Direct mechanocatalysis: palladium as milling media and catalyst in the mechanochemical Suzuki polymerization. Angew. Chem. Int. Ed. 58, 18942–18947 (2019).
Fulmer, D. A., Shearouse, W. C., Medonza, S. T. & Mack, J. Solvent-free Sonogashira coupling reaction via high speed ball milling. Green Chem. 11, 1821–1825 (2009).
Friščić, T. et al. Ion‐ and liquid‐assisted grinding: improved mechanochemical synthesis of metal–organic frameworks reveals salt inclusion and anion templating. Angew. Chem. Int. Ed. 49, 712–715 (2009).
Hasa, D., Schneider Rauber, G., Voinovich, D. & Jones, W. Cocrystal formation through mechanochemistry: from neat and liquid-assisted grinding to polymer-assisted grinding. Angew. Chem. Int. Ed. 54, 7371–7375 (2015).
Brekalo, I. et al. Use of a “shoe-last” solid-state template in the mechanochemical synthesis of high-porosity RHO-zinc imidazolate. J. Am. Chem. Soc. 140, 10104–10108 (2018).
Arhangelskis, M. et al. Mechanochemical reactivity inhibited, prohibited and reversed by liquid additives: examples from crystal-form screens. Chem. Sci. 12, 3264–3269 (2021).
Belenguer, A. M., Lampronti, G. I., Wales, D. J. & Sanders, J. K. M. Direct observation of intermediates in a thermodynamically controlled solid-state dynamic covalent reaction. J. Am. Chem. Soc. 136, 16156–16166 (2014).
Tireli, M. et al. Mechanochemical reactions studied by in situ Raman spectroscopy: base catalysis in liquid-assisted grinding. Chem. Commun. 51, 8058–8061 (2015).
Brekalo, I. et al. Scale-up of agrochemical urea-gypsum cocrystal synthesis using thermally controlled mechanochemistry. ACS Sustain. Chem. Eng. 10, 6743–6754 (2022).
Užarević, K., Halasz, I. & Friščić, T. Real-time and in situ monitoring of mechanochemical reactions: a new playground for all chemists. J. Phys. Chem. Lett. 6, 4129–4140 (2015).
Friščić, T. et al. Real-time and in situ monitoring of mechanochemical milling reactions. Nat. Chem. 5, 66–73 (2013).
de Oliveira, P. F. M. et al. Tandem X-ray absorption spectroscopy and scattering for in situ time-resolved monitoring of gold nanoparticle mechanosynthesis. Chem. Commun. 56, 10329–10332 (2020).
Gracin, D., Štrukil, V., Friščić, T., Halasz, I. & Užarević, K. Laboratory real-time and in situ monitoring of mechanochemical milling reactions by Raman spectroscopy. Angew. Chem. Int. Ed. 53, 6193–6197 (2014).
Lukin, S., Užarević, K. & Halasz, I. Raman spectroscopy for real-time and in situ monitoring of mechanochemical milling reactions. Nat. Protoc. 16, 3492–3521 (2021).
Pladevall, B. S., de Aguirre, A. & Maseras, F. Understanding ball milling mechanochemical processes with DFT calculations and microkinetic modeling. ChemSusChem 14, 2763–2768 (2021).
Delogu, F. Molecular dynamics of collisions between rough surfaces. Phys. Rev. B 82, 205415 (2010).
Chen, Z., Vazirisereshk, M. R., Khajeh, A., Martini, A. & Kim, S. H. Effect of atomic corrugation on adhesion and friction: a model study with graphene step edges. J. Phys. Chem. Lett. 10, 6455–6461 (2019).
Ferguson, M. et al. Insights into mechanochemical reactions at the molecular level: simulated indentations of aspirin and meloxicam crystals. Chem. Sci. 10, 2924–2929 (2019).
Ewers, B. W. & Batteas, J. D. Utilizing atomistic simulations to map pressure distributions and contact areas in molecular adlayers within nanoscale surface-asperity junctions: a demonstration with octadecylsilane-functionalized silica interfaces. Langmuir 30, 11897–11905 (2014).
Butyagin, P. Y. The nature of the mechanical degradation of polymethylmethacrylate. Polym. Sci. USSR 9, 149–158 (1967).
Užarević, K. et al. Enthalpy vs. friction: heat flow modelling of unexpected temperature profiles in mechanochemistry of metal–organic frameworks. Chem. Sci. 9, 2525–2532 (2018).
Friščić, T. & Jones, W. Recent advances in understanding the mechanism of cocrystal formation via grinding. Cryst. Growth Des. 9, 1621–1637 (2009).
Bowden, F. P., Stone, M. A. & Tudor, G. K. Hot spots on rubbing surfaces and the detonation of explosives by friction. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 188, 329–349 (1947).
Fox, P. G. Mechanically initiated chemical reactions in solids. J. Mater. Sci. 10, 340–360 (1975).
Thiessen, P. A., Meyer K. & Heinicke, G. Grundlagen der Tribochemie Ch. 1 (Akademie Verlag, 1967).
Užarević, K. et al. Exploring the effect of temperature on a mechanochemical reaction by in situ synchrotron powder X-ray diffraction. Cryst. Growth Des. 16, 2342–2347 (2016).
Lea, M. C. Disruption of the silver haloid molecule by mechanical force. Am. J. Sci. s3-43, 527–531 (1892).
Lea, M. C. On endothermic reactions effected by mechanical force. Am. J. Sci. s3-46, 241–244 (1893).
Lea, M. C. Transformations of mechanical into chemical energy; part III, action of shearing stress continued. Am. J. Sci. s3-47, 377–382 (1894).
Boldyrev, V. V., Avakumow, E. G., Strugowa, L. I., Harenz, H. & Heinicke, G. Zur Tribochemischen Zersetzung von Alkali‐Bromaten und‐Nitraten. Z. Anorg. Allg. Chem. 393, 152–158 (1972).
Milanović, I. et al. Mechanochemical synthesis and thermal dehydrogenation of novel calcium-containing bimetallic amidoboranes. ACS Sustain. Chem. Eng. 9, 2089–2099 (2021).
Takacs, L. Self-sustaining reactions as a tool to study mechanochemical activation. Faraday Discuss. 170, 251–265 (2014).
Macfadyen, A. On the influence of the prolonged action of the temperature of liquid air on micro-organisms, and on the effect of mechanical trituration at the temperature of liquid air on photogenic bacteria. Proc. R. Soc. Lond. 71, 76–77 (1902).
Cells, O. & Barnard, M. On a method of disintegrating bacterial and other organic cells. Proc. R. Soc. Lond. Ser. B Biol. Sci. 84, 57–66 (1911).
Mudd, S., Shaw, C. H., Czarnetzky, E. J. & Flosdorf, E. W. A low temperature ball-mill for the liberation of labile cellular products. J. Immunol. 32, 483–489 (1937).
Kaupp, G., Boy, J. & Schmeyers, J. Iminiumsalze in quantitativen Gas/Festköper- und Festkörper/Festkörper-Reaktionen. J. Prakt. Chem. Chemiker-Ztg. 340, 346–355 (1998).
Kaupp, G., Reza Naimi-Jamal, M. & Schmeyers, J. Solvent-free Knoevenagel condensations and Michael additions in the solid state and in the melt with quantitative yield. Tetrahedron 59, 3753–3760 (2003).
Andersen, J. & Mack, J. Insights into mechanochemical reactions at targetable and stable, sub-ambient temperatures. Angew. Chem. Int. Ed. 57, 13062–13065 (2018).
Andersen, J., Brunemann, J. & Mack, J. Exploring stable, sub-ambient temperatures in mechanochemistry via a diverse set of enantioselective reactions. React. Chem. Eng. 4, 1229–1236 (2019).
Andersen, J. M. & Mack, J. Decoupling the Arrhenius equation via mechanochemistry. Chem. Sci. 8, 5447–5453 (2017).
André, V. et al. Mechanosynthesis of the metallodrug bismuth subsalicylate from Bi2O3 and structure of bismuth salicylate without auxiliary organic ligands. Angew. Chem. Int. Ed. 50, 1433–7851 (2011).
Seo, T., Toyoshima, N., Kubota, K. & Ito, H. Tackling solubility issues in organic synthesis: solid-state cross-coupling of insoluble aryl halides. J. Am. Chem. Soc. 143, 6165–6175 (2021).
Takahashi, R. et al. Mechanochemical synthesis of magnesium-based carbon nucleophiles in air and their use in organic synthesis. Nat. Commun. 12, 6691 (2021).
Gao, P., Jiang, J., Maeda, S., Kubota, K. & Ito, H. Mechanochemically generated calcium‐based heavy Grignard reagents and their application to carbon–carbon bond‐forming reactions. Angew. Chem. Int. Ed. 61, e202207118 (2022).
Kubota, K., Endo, T., Uesugi, M., Hayashi, Y. & Ito, H. Solid-state C–N cross-coupling reactions with carbazoles as nitrogen nucleophiles using mechanochemistry. ChemSusChem 15, e202102132 (2022).
Cindro, N., Tireli, M., Karadeniz, B., Mrla, T. & Užarević, K. Investigations of thermally controlled mechanochemical milling reactions. ACS Sustain. Chem. Eng. 7, 16301–16309 (2019).
Stolar, T. et al. Mechanochemical prebiotic peptide bond formation. Angew. Chem. Int. Ed. 60, 12727–12731 (2021).
Linberg, K., Röder, B., Al-Sabbagh, D., Emmerling, F. & Michalchuk, A. L. Controlling polymorphism in molecular cocrystals by variable temperature ball milling. Faraday Discuss. https://doi.org/10.1039/D2FD00115B (2022).
Crawford, D. E. & Casaban, J. Recent developments in mechanochemical materials synthesis by extrusion. Adv. Mater. 28, 5747–5754 (2016).
Crawford, D. E., Miskimmin, C. K. G., Albadarin, A. B., Walker, G. & James, S. L. Organic synthesis by twin screw extrusion (TSE): continuous, scalable and solvent-free. Green Chem. 19, 1507–1518 (2017).
Crawford, D. et al. Synthesis by extrusion: continuous, large-scale preparation of MOFs using little or no solvent. Chem. Sci. 6, 1645–1649 (2015).
Karadeniz, B. et al. Benign by design: green and scalable synthesis of zirconium UiO-metal–organic frameworks by water-assisted mechanochemistry. ACS Sustain. Chem. Eng. 6, 15841–15849 (2018).
Cintas, P., Cravotto, G., Barge, A. & Martina, K. in Polymer Mechanochemistry (ed. Boulatov, R.) 239–284 (Springer, 2014).
Giannakoudakis, D. A., Chatel, G. & Colmenares, J. C. in Heterogeneous Photocatalysis (eds Muñoz-Batista, M., Navarrete Muñoz, A. & Luque, R.) 29–70 (Springer, 2020).
Cravotto, G., Gaudino, E. C. & Cintas, P. On the mechanochemical activation by ultrasound. Chem. Soc. Rev. 42, 7521–7534 (2013).
Chatel, G. & Varma, R. S. Ultrasound and microwave irradiation: contributions of alternative physicochemical activation methods to green chemistry. Green Chem. 21, 6043–6050 (2019).
Santos, H. M.; Lodeiro, C. & Capelo-Martínez, J.-L. in Ultrasound in Chemistry: Analytical Applications (ed. Capelo-Martínez, J.-L.) Ch. 1 (Wiley, 2009).
Mordyuk, B. N. & Prokopenko, G. I. Mechanical alloying of powder materials by ultrasonic milling. Ultrasonics 42, 43–46 (2004).
Chen, D. & Xiao, T. One-step synthesis of Zn to single-phase nanocrystalline ZnO by solid-liquid reaction ball milling assisted by ultrasonic wave. J. Am. Ceram. Soc. 93, 2675–2678 (2010).
Suslick, K. S. Applications of ultrasound to materials chemistry. MRS Bull. 20, 29–34 (1995).
Kozlov, A. V., Mordyuk, B. N. & Prokopenko G. I. Device for production of powder materials. Ukraine patent 59770A. https://sis.ukrpatent.org/en/search/detail/370903/ (2002).
Perekos, A. O. et al. Structural state and magnetic properties of the nanocrystalline Ni fabricated in a ultrasonic ball mill. Metallofiz. Noveishie Tekhnologii 29, 211–223 (2007).
Perekos, А. Е. et al. Study of the process of gas emission from Ni nanocrystalline powders fabricated by ball grinding in ultrasonic mill. Metallofiz. Noveishie Tekhnologii 33, 93–103 (2011).
Nadutov, V. M. et al. Influence of ultrasonic processing in a ball mill on phase-structural characteristics of superfine powder blends of copper with iron and cobalt. Metallofiz. Noveishie Tekhnologii 40, 501–514 (2018).
Nadutov, V. M., Mordyuk, B. N., Volosevich, P. Y., Svistunov, E. A. & Perizhnyak, A. V. Effect of graphite on the degree of grinding and the structure of α-Fe powder in an ultrasonic mill. Phys. Met. Metallogr. 104, 415–424 (2007).
Nadutov, V. M. et al. Investigation of process of layering of the solid solutions formed by ultrasonic milling of coarse-grained powder blends of copper with cobalt and copper with iron, and its influence on their structure–phase state and magnetic properties. Metallofiz. Noveishie Tekhnologii 40, 1185–1199 (2018).
Nadutov, V. M. et al. Thermal stability of solid solutions formed by ultrasonic milling of Cu–Co and Cu–Fe powder mixtures. Ukr. J. Phys. 62, 685–691 (2017).
Nadutov, V. M. et al. Structure and magnetic properties of the Cu–Co and Cu–Fe nanopowders obtained in ultrasonic ball mill. Metallofiz. Noveishie Tekhnologii 39, 525–539 (2017).
Chen, D., Liu, H. Y. & Xia, S. R. One-step decomposition of basic carbonates into single-phase crystalline metallic oxides nanoparticle by ultrasonic wave-assisted ball milling technology. Ceram. Int. 38, 821–825 (2012).
Chen, D., Liu, H. Y.- & Li, L. One-step synthesis of manganese ferrite nanoparticles by ultrasonic wave-assisted ball milling technology. Mater. Chem. Phys. 134, 921–924 (2012).
Yuan, Z., Chen, Z. H., Chen, D. & Kang, Z. T. Analyses of factors affecting nickel ferrite nanoparticles synthesis in ultrasound-assisted aqueous solution ball milling. Ultrason. Sonochem. 22, 188–197 (2015).
Luo, Y., Chen, D., Wei, F. & Liang, Z. Synthesis of Cu-BTC metal-organic framework by ultrasonic wave-assisted ball milling with enhanced Congo red removal property. ChemistrySelect 3, 11435–11440 (2018).
Stolar, T. et al. In situ monitoring of the mechanosynthesis of the archetypal metal–organic framework HKUST-1: effect of liquid additives on the milling reactivity. Inorg. Chem. 56, 6599–6608 (2017).
Tanaka, R. et al. Verification of the mixing processes of the active pharmaceutical ingredient, excipient and lubricant in a pharmaceutical formulation using a resonant acoustic mixing technology. RSC Adv. 6, 87049–87057 (2016).
Rumeau, N., Threlfall, D. & Wilmet, A. in Proc. Insensitive Munitions & Energetic Materials Technology Symposium (IMEMTS) 1–10 (2015).
Titi, H. M., Do, J. L., Howarth, A. J., Nagapudi, K. & Friščić, T. Simple, scalable mechanosynthesis of metal–organic frameworks using liquid-assisted resonant acoustic mixing (LA-RAM). Chem. Sci. 11, 7578–7584 (2020).
Michalchuk, A. A. L. et al. Ball-free mechanochemistry: in situ real-time monitoring of pharmaceutical co-crystal formation by resonant acoustic mixing. Chem. Commun. 54, 4033–4036 (2018).
Nagapudi, K., Umanzor, E. Y. & Masui, C. High-throughput screening and scale-up of cocrystals using resonant acoustic mixing. Int. J. Pharm. 521, 337–345 (2017).
Am Ende, D. J., Anderson, S. R. & Salan, J. S. Development and scale-up of cocrystals using resonant acoustic mixing. Org. Process. Res. Dev. 18, 331–341 (2014).
Andrews, M. R., Collet, C., Wolff, A. & Hollands, C. Resonant acoustic® mixing: processing and safety. Propellants Explos. Pyrotech. 45, 77–86 (2020).
Anderson, S. R., Am Ende, D. J., Salan, J. S. & Samuels, P. Preparation of an energetic-energetic cocrystal using resonant acoustic mixing. Propellants Explos. Pyrotech. 39, 637–640 (2014).
Zhang, J. & Shreeve, J. M. Time for pairing: cocrystals as advanced energetic materials. CrystEngComm 18, 6124–6133 (2016).
Green, N. J., Xu, J. & Sutherland, J. D. Illuminating life’s origins: UV photochemistry in abiotic synthesis of biomolecules. J. Am. Chem. Soc. 143, 7219–7236 (2021).
Hoffmann, N. Photochemical reactions as key steps in organic synthesis. Chem. Rev. 108, 1052–1103 (2008).
Atkinson, M. B. J. et al. General application of mechanochemistry to templated solid-state reactivity: rapid and solvent-free access to crystalline supermolecules. Chem. Commun. 5713–5715 (2008).
Ma, D.-Y. & Wärnmark, K. Mechanoassisted supramolecular catalysis in solid state synthesis. ChemCatChem 2, 1059–1060 (2010).
Yelgaonkar, S. P., Swenson, D. C. & MacGillivray, L. R. Supramolecular chemistry under mechanochemical conditions: a small molecule template generated and integrated into a molecular-to-supramolecular and back-to-molecular cascade reaction. Chem. Sci. 11, 3569–3573 (2020).
Hernández, J. G. Mechanochemical borylation of aryldiazonium salts; merging light and ball milling. Beilstein J. Org. Chem. 13, 1463–1469 (2017).
Toda, F., Tanaka, K. & Sekikawa, A. Host–guest complex formation by a solid–solid reaction. J. Chem. Soc. Chem. Commun. https://doi.org/10.1039/C39870000279 (1987).
Sokolov, A. N., Bučar, D.-K., Baltrusaitis, J., Gu, S. X. & MacGillivray, L. R. Supramolecular catalysis in the organic solid state through dry grinding. Angew. Chem. Int. Ed. 49, 4273–4277 (2010).
Stojaković, J., Farris, B. S. & MacGillivray, L. R. Vortex grinding for mechanochemistry: application for automated supramolecular catalysis and preparation of a metal–organic framework. Chem. Commun. 48, 7958–7960 (2012).
Obst, M. & König, B. Solvent-free, visible-light photocatalytic alcohol oxidations applying an organic photocatalyst. Beilstein J. Org. Chem. 12, 2358–2363 (2016).
Štrukil, V. & Sajko, I. Mechanochemically-assisted solid-state photocatalysis (MASSPC). Chem. Commun. 53, 9101–9104 (2017).
Schotten, C. et al. Making electrochemistry easily accessible to the synthetic chemist. Green Chem. 22, 3358–3375 (2020).
Zhu, C., Ang, N. W. J., Meyer, T. H., Qiu, Y. & Ackermann, L. Organic electrochemistry: molecular syntheses with potential. ACS Cent. Sci. 7, 415–431 (2021).
Calka, A. & Wexler, D. Mechanical milling assisted by electrical discharge. Nature 419, 147–151 (2002).
Calka, A. & Wexler, D. Processing of materials by electric discharge assisted mechanical milling. Mater. Sci. Forum 674, 29–39 (2011).
Zhu, M., Dai, L. Y., Gu, N. S., Cao, B. & Ouyang, L. Z. Synergism of mechanical milling and dielectric barrier discharge plasma on the fabrication of nano-powders of pure metals and tungsten carbide. J. Alloys Compd. 478, 624–629 (2009).
Borcia, G., Anderson, C. A. & Brown, N. M. D. Dielectric barrier discharge for surface treatment: application to selected polymers in film and fibre form. Plasma Sources Sci. Technol. 12, 335–344 (2003).
Ouyang, L., Cao, Z., Wang, H., Hu, R. & Zhu, M. Application of dielectric barrier discharge plasma-assisted milling in energy storage materials – a review. J. Alloys Compd. 691, 422–435 (2017).
Cao, Z. et al. Dual-tuning effects of In, Al, and Ti on the thermodynamics and kinetics of Mg85In5Al5Ti5 alloy synthesized by plasma milling. J. Alloys Compd. 623, 354–358 (2015).
Schumacher, C., Hernández, J. G. & Bolm, C. Electro‐mechanochemical atom transfer radical cyclizations using piezoelectric BaTiO3. Angew. Chem. Int. Ed. 59, 16357–16360 (2020).
Kubota, K., Pang, Y., Miura, A. & Ito, H. Redox reactions of small organic molecules using ball milling and piezoelectric materials. Science 366, 1500–1504 (2019).
Pang, Y., Won Lee, J., Kubota, K. & Ito, H. Solid-state radical C–H trifluoromethylation reactions using ball milling and piezoelectric materials. Angew. Chem. Int. Ed. 59, 2570–22576 (2020).
Acknowledgements
This work has been supported in part by the ‘Research Cooperability’ Program of the Croatian Science Foundation funded by the European Union from the European Social Fund under the Operational Programme Efficient Human Resources 2014–2020, through grant PZS-2019-02-4129. The authors acknowledge the Croatian Science Foundation (grant no. IP-2020-02-4702) for financial support. The authors thank Ivan Kulcsár for help with slow-motion photography.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to discussion of content, writing and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
K.U. is a shareholder in InSolido Technologies, which produces milling reactors.
Peer review
Peer review information
Nature Reviews Chemistry thanks Michael Ferguson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Low-frequency sonic mixing technology: www.energy.gov/eere/amo/low-frequency-sonic-mixing-technology
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martinez, V., Stolar, T., Karadeniz, B. et al. Advancing mechanochemical synthesis by combining milling with different energy sources. Nat Rev Chem 7, 51–65 (2023). https://doi.org/10.1038/s41570-022-00442-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-022-00442-1
This article is cited by
-
Mechanochemical synthesis of organoselenium compounds
Nature Communications (2024)
-
Mechanochemical synthesis of halogenated heterocyclic compounds
Chemistry of Heterocyclic Compounds (2023)