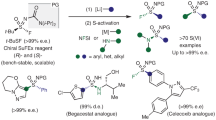
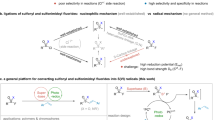
Abstract
The advent of sulfur(vi)-fluoride exchange (SuFEx) processes as transformations with click-like reactivity has invigorated research into electrophilic species featuring a sulfur–fluorine bond. Among these, sulfonyl fluorides have emerged as the workhorse functional group, with diverse applications being reported. Sulfonyl fluorides are used as electrophilic warheads by both medicinal chemists and chemical biologists. The balance of reactivity and stability that is so attractive for these applications, particularly the resistance of sulfonyl fluorides to hydrolysis under physiological conditions, has provided opportunities for synthetic chemists. New synthetic approaches that start with sulfur-containing substrates include the activation of sulfonamides using pyrilium salts, the deoxygenation of sulfonic acids, and the electrochemical oxidation of thiols. Employing non-sulfur-containing substrates has led to the development of transition-metal-catalysed processes based on palladium, copper and nickel, as well as the use of SO2F2 gas as an electrophilic hub. Selectively manipulating molecules that already contain a sulfonyl fluoride group has also proved to be a popular tactic, with metal-catalysed processes again at the fore. Finally, coaxing sulfonyl fluorides to engage with nucleophiles, when required, and under suitable reaction conditions, has led to new activation methods. This Review provides an overview of the challenges in the efficient synthesis and manipulation of these intriguing functional groups.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Steinkopf, W. Über aromatische sulfofluoride. J. Prakt. Chem. 117, 1–82 (1927).
Dong, J., Krasnova, L., Finn, M. G. & Sharpless, K. B. Sulfur(vi) fluoride exchange (SuFEx): another good reaction for click chemistry. Angew. Chem. Int. Ed. 53, 9430–9448 (2014). This is the report that sparked the current interest in S(VI)-F-containing electrophiles.
Steinkopf, W. & Jaeger, P. Über aromatische sulfofluoride. II. Mitteilung. J. Prakt. Chem. 128, 63–88 (1930).
Davies, W. & Dick, J. H. CCLXXXVI. — Aromatic sulphonyl fluorides: a convenient method of preparation. J. Chem. Soc. 1931, 2104–2109 (1931).
Wiberg, E. & Holleman, A. F. Inorganic Chemistry (Academic, 2001).
Takacs, G. A. Heats of formation and bond dissociation energies of some simple sulfur- and halogen-containing molecules. J. Chem. Eng. Data 23, 174–175 (1978).
Wray, K. L. & Feldman, E. V. Shock tube study of the decomposition kinetics of SO2F2. J. Chem. Phys. 54, 3445–3449 (1971).
Cady, G. H. & Misra, S. Hydrolysis of sulfuryl fluoride. Inorg. Chem. 13, 837–841 (1974).
Aberlin, M. E. & Bunton, C. A. Spontaneous hydrolysis of sulfonyl fluorides. J. Org. Chem. 35, 1825–1828 (1970).
Swain, C. G. & Scott, C. B. Rates of solvolysis of some alkyl fluorides and chlorides1. J. Am. Chem. Soc. 75, 246–248 (1953).
Bogolubsky, A. V. et al. Sulfonyl fluorides as alternative to sulfonyl chlorides in parallel synthesis of aliphatic sulfonamides. ACS Comb. Sci. 16, 192–197 (2014).
Chinthakindi, P. K. & Arvidsson, P. I. Sulfonyl fluorides (SFs): more than click reagents? Eur. J. Org. Chem. 3648–3666 (2018).
Barrow, A. S. et al. The growing applications of SuFEx click chemistry. Chem. Soc. Rev. 48, 4731–4758 (2019). This excellent review focuses on the reactivity of sulfonyl fluorides in click applications.
Ball, N. D. in Emerging Fluorinated Motifs (eds Cahard, J. & Ma, J.-A.) 621–674 (Wiley, 2020).
Narayanan, A. & Jones, L. H. Sulfonyl fluorides as privileged warheads in chemical biology. Chem. Sci. 6, 2650–2659 (2015). This review provides a good overview of the use of sulfonyl fluorides as covalent binding groups in chemical biology applications.
Mukherjee, H. et al. A study of the reactivity of S(vi)–F containing warheads with nucleophilic amino-acid side chains under physiological conditions. Org. Biomol. Chem. 15, 9685–9695 (2017).
Shannon, D. A. et al. Sulfonyl fluoride analogues as activity-based probes for serine proteases. ChemBioChem 13, 2327–2330 (2012).
Fahrney, D. E. & Gold, A. M. Sulfonyl fluorides as inhibitors of esterases. I. Rates of reaction with acetylcholinesterase, α-chymotrypsin, and trypsin. J. Am. Chem. Soc. 85, 997–1000 (1963).
Markwardt, F., Drawert, J. & Walsmann, P. Synthetic low molecular weight inhibitors of serum kallikrein. Biochem. Pharmacol. 23, 2247–2256 (1974).
Kamps, M. P., Taylor, S. S. & Sefton, B. M. Direct evidence that oncogenic tyrosine kinases and cyclic AMP-dependent protein kinase have homologous ATP-binding sites. Nature 310, 589–592 (1984).
Hett, E. C. et al. Rational targeting of active-site tyrosine residues using sulfonyl fluoride probes. ACS Chem. Biol. 10, 1094–1098 (2015).
Emsley, J. & Overill, R. E. Defining the bond energy of a strong hydrogen bond. Chem. Phys. Lett. 65, 616–617 (1979).
Larson, J. W. & McMahon, T. B. Gas-phase bifluoride ion. An ion cyclotron resonance determination of the hydrogen bond energy in fluoride ion (FHF-) from gas-phase fluoride transfer equilibrium measurements. J. Am. Chem. Soc. 104, 5848–5849 (1982).
Mukherjee, P. et al. Sulfonamide synthesis via calcium triflimide activation of sulfonyl fluorides. Org. Lett. 20, 3943–3947 (2018).
Mahapatra, S. et al. SuFEx activation with Ca(NTf2)2: a unified strategy to access sulfamides, sulfamates, and sulfonamides from S(vi) fluorides. Org. Lett. 22, 4389–4394 (2020). This paper documents a powerful method for the reaction of sulfonyl fluorides, and related S-F electrophiles, with amines.
Chatgilialoglu, C., Griller, D. & Guerra, M. Experimental and theoretical approaches to the optical absorption spectra of sulfonyl radicals. J. Phys. Chem. 91, 3747–3750 (1987).
Chatgilialoglu, C., Griller, D., Kanabus-Kaminska, J. M. & Lossing, F. P. Sulfur–chlorine bond dissociation enthalpies in methane- and benzene-sulfonyl chlorides. J. Chem. Soc. Perkin Trans. 2, 357–360 (1994).
Hirsch, E., Hünig, S. & Reißig, H.-U. Darstellung von (2,2-Dimethyl-1-methylenpropyl)-methansulfonat und -trifluoracetat. Chem. Ber. 115, 399–401 (1982).
Kwon, J. & Kim, B. M. Synthesis of arenesulfonyl fluorides via sulfuryl fluoride incorporation from arynes. Org. Lett. 21, 428–433 (2019).
Lee, C., Ball, N. D. & Sammis, G. M. One-pot fluorosulfurylation of Grignard reagents using sulfuryl fluoride. Chem. Commun. 55, 14753–14756 (2019).
Smedley, C. J. et al. Diversity oriented clicking (DOC): divergent synthesis of sufexable pharmacophores from 2-substituted-alkynyl-1-sulfonyl fluoride (SASF) hubs. Angew. Chem. Int. Ed. 59, 12460–12469 (2020).
So, C. M. & Kwong, F. Y. Palladium-catalyzed cross-coupling reactions of aryl mesylates. Chem. Soc. Rev. 40, 4963–4972 (2011).
Nie, X. et al. Radical fluorosulfonylation: accessing alkenyl sulfonyl fluorides from alkenes. Angew. Chem. Int. Ed. 60, 3956–3960 (2021). An example of generating and using the fluorosulfonyl radical in a synthetically useful transformation.
Boiko, V. N. et al. A convenient synthetic route to 2,4,6-tris(chlorosulfonyl)- and 2,4,6-tris(fluorosulfonyl)phenol, aniline and chlorobenzene. J. Fluor. Chem. 132, 1219–1226 (2011).
Davies, W. & Dick, J. H. 57. Aliphatic sulphonyl fluorides. J. Chem. Soc. 1932, 483–486 (1932).
Bianchi, T. A. & Cate, L. A. Phase transfer catalysis. Preparation of aliphatic and aromatic sulfonyl fluorides. J. Org. Chem. 42, 2031–2032 (1977).
Kang, S. O., Powell, D., Day, V. W. & Bowman-James, K. Trapped bifluoride. Angew. Chem. Int. Ed. 45, 1921–1925 (2006).
Beaman, A. G. & Robins, R. K. Potential purine antagonists. XXVII. Synthesis and reactions of some purinesulfonyl fluorides. J. Am. Chem. Soc. 83, 4038–4044 (1961).
Brown, D. J. & Hoskins, J. A. Simple pyrimidines. Part XIV. The formation and reactions of some derivatives of simple pyrimidinesulphonic acids. J. Chem. Soc. Perkin Trans. 1, 522–527 (1972).
Narayan, S. et al. “On water”: unique reactivity of organic compounds in aqueous suspension. Angew. Chem. Int. Ed. 44, 3275–3279 (2005).
Caddick, S., Wilden, J. D., Bush, H. D., Wadman, S. N. & Judd, D. B. A new route to sulfonamides via intermolecular radical addition to pentafluorophenyl vinylsulfonate and subsequent aminolysis. Org. Lett. 4, 2549–2551 (2002).
Caddick, S., Wilden, J. D. & Judd, D. B. Observations on the reactivity of pentafluorophenyl sulfonate esters. Chem. Commun. 21, 2727–2728 (2005).
Vedovato, V., Talbot, E. P. A. & Willis, M. C. Copper-catalyzed synthesis of activated sulfonate esters from boronic acids, DABSO, and pentafluorophenol. Org. Lett. 20, 5493–5496 (2018).
Zincke, T. & Frohneberg, W. Über Dithiohydrochinon. Ber. Dtsch. Chem. Ges. 42, 2721–2736 (1909).
Douglass, I. B. & Johnson, T. B. The interaction of chlorine with different types of organic sulfur compounds. J. Am. Chem. Soc. 60, 1486–1489 (1938).
Caldwell, W. T. & Kornfeld, E. C. Substituted 2-sulfonamido-5-aminopyridines. J. Am. Chem. Soc. 64, 1695–1698 (1942).
Wright, S. W. & Hallstrom, K. N. A convenient preparation of heteroaryl sulfonamides and sulfonyl fluorides from heteroaryl thiols. J. Org. Chem. 71, 1080–1084 (2006).
Wang, L. & Cornella, J. A unified strategy for arylsulfur(vi) fluorides from aryl halides: access to Ar-SOF3 compounds. Angew. Chem. Int. Ed. 59, 23510–23515 (2020). This shows oxidative chlorination followed by fluorination, using a convenient more sustainable chlorinating reagent.
Kim, J.-G. & Jang, D. O. A convenient, one-pot procedure for the preparation of acyl and sulfonyl fluorides using Cl3CCN, Ph3P, and TBAF(t-BuOH)4. Synlett 2010, 3049–3052 (2010).
Jiang, Y., Alharbi, N. S., Sun, B. & Qin, H.-L. Facile one-pot synthesis of sulfonyl fluorides from sulfonates or sulfonic acids. RSC Adv. 9, 13863–13867 (2019).
Gómez-Palomino, A. & Cornella, J. Selective late-stage sulfonyl chloride formation from sulfonamides enabled by Pyry-BF4. Angew. Chem. Int. Ed. 58, 18235–18239 (2019).
Pérez-Palau, M. & Cornella, J. Synthesis of sulfonyl fluorides from sulfonamides. Eur. J. Org. Chem. 2497–2500 (2020). This report shows how widely available primary sulfonamides can be used as substrates to access sulfonyl fluorides.
Segall, Y., Quistad, G. B. & Casida, J. E. Cannabinoid CB1 receptor chemical affinity probes: methods suitable for preparation of isopropyl [11,12-3H]dodecylfluorophosphonate and [11,12-3H]dodecanesulfonyl fluoride. Synth. Commun. 33, 2151–2159 (2003).
Brouwer, A. J., Ceylan, T., Linden, T. V. D. & Liskamp, R. M. J. Synthesis of β-aminoethanesulfonyl fluorides or 2-substituted taurine sulfonyl fluorides as potential protease inhibitors. Tetrahedron Lett. 50, 3391–3393 (2009).
Kirihara, M., Naito, S., Ishizuka, Y., Hanai, H. & Noguchi, T. Oxidation of disulfides with Selectfluor™: concise syntheses of thiosulfonates and sulfonyl fluorides. Tetrahedron Lett. 52, 3086–3089 (2011).
Kirihara, M. et al. Oxidation of disulfides with electrophilic halogenating reagents: concise methods for preparation of thiosulfonates and sulfonyl halides. Tetrahedron 70, 2464–2471 (2014).
Laudadio, G. et al. Sulfonyl fluoride synthesis through electrochemical oxidative coupling of thiols and potassium fluoride. J. Am. Chem. Soc. 141, 11832–11836 (2019). This electrochemistry approach uses simple starting materials — thiols and fluoride — to construct sulfonyl fluorides, with potential sustainable advantages.
Banks, R. E., Besheesh, M. K., Mohialdin-Khaffaf, S. N. & Sharif, I. N-Halogeno compounds. Part 18. 1-Alkyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane salts: user-friendly site-selective electrophilic fluorinating agents of the N-fluoroammonium class. J. Chem. Soc. Perkin Trans. 1, 2069–2076 (1996).
Toulgoat, F., Langlois, B. R., Médebielle, M. & Sanchez, J.-Y. An efficient preparation of new sulfonyl fluorides and lithium sulfonates. J. Org. Chem. 72, 9046–9052 (2007).
Emmett, E. Development of Catalytic Methods to Exploit Sulfur Dioxide in Organic Synthesis. Thesis, Univ. Oxford (2014).
Davies, A. T., Curto, J. M., Bagley, S. W. & Willis, M. C. One-pot palladium-catalyzed synthesis of sulfonyl fluorides from aryl bromides. Chem. Sci. 8, 1233–1237 (2017).
Tribby, A. L., Rodriguez, I., Shariffudin, S. & Ball, N. D. Pd-catalyzed conversion of aryl iodides to sulfonyl fluorides using SO2 surrogate DABSO and Selectfluor. J. Org. Chem. 82, 2294–2299 (2017). This, and the preceding paper, show that readily available non-sulfur containing substrates, aryl halides, can be converted into sulfonyl fluorides.
Nguyen, B., Emmett, E. J. & Willis, M. C. Palladium-catalyzed aminosulfonylation of aryl halides. J. Am. Chem. Soc. 132, 16372–16373 (2010).
Richards-Taylor, C. S., Blakemore, D. C. & Willis, M. C. One-pot three-component sulfone synthesis exploiting palladium-catalysed aryl halide aminosulfonylation. Chem. Sci. 5, 222–228 (2014).
Emmett, E. J., Hayter, B. R. & Willis, M. C. Palladium-catalyzed synthesis of ammonium sulfinates from aryl halides and a sulfur dioxide surrogate: a gas- and reductant-free process. Angew. Chem. Int. Ed. 53, 10204–10208 (2014).
Deeming, A. S., Russell, C. J. & Willis, M. C. Palladium(ii)-catalyzed synthesis of sulfinates from boronic acids and DABSO: a redox-neutral, phosphine-free transformation. Angew. Chem. Int. Ed. 55, 747–750 (2016).
Johnson, M. W. et al. Application of fundamental organometallic chemistry to the development of a gold-catalyzed synthesis of sulfinate derivatives. Angew. Chem. Int. Ed. 53, 4404–4407 (2014).
Chen, Y. & Willis, M. C. Copper(i)-catalyzed sulfonylative Suzuki–Miyaura cross-coupling. Chem. Sci. 8, 3249–3253 (2017).
Lo, P. K. T., Chen, Y. & Willis, M. C. Nickel(ii)-catalyzed synthesis of sulfinates from aryl and heteroaryl boronic acids and the sulfur dioxide surrogate DABSO. ACS Catal. 9, 10668–10673 (2019).
Lou, T. S.-B., Bagley, S. W. & Willis, M. C. Cyclic alkenylsulfonyl fluorides: palladium-catalyzed synthesis and functionalization of compact multifunctional reagents. Angew. Chem. Int. Ed. 58, 18859–18863 (2019).
Liu, Y. et al. Arenesulfonyl fluoride synthesis via copper-catalyzed fluorosulfonylation of arenediazonium salts. Org. Lett. 22, 2281–2286 (2020).
Liu, S., Huang, Y., Xu, X.-H. & Qing, F.-L. Fluorosulfonylation of arenediazonium tetrafluoroborates with Na2S2O5 and N-fluorobenzenesulfonimide. J. Fluor. Chem. 240, 109653 (2020).
Lin, Q. et al. Arenesulfonyl fluoride synthesis via copper-free Sandmeyer-type fluorosulfonylation of arenediazonium salts. Chin. J. Chem. 38, 1107–1110 (2020).
Zhong, T. et al. Copper-free Sandmeyer-type reaction for the synthesis of sulfonyl fluorides. Org. Lett. 22, 3072–3078 (2020).
Louvel, D. et al. Metal-free visible-light synthesis of arylsulfonyl fluorides: scope and mechanism. Chemistry 27, 8704–8708 (2021).
Ykman, P. & Hall, H. K. One-step conversion of alkoxytrimethylsilanes to alkyl benzenesulfonates. J. Organomet. Chem. 116, 153–159 (1976).
Gembus, V., Marsais, F. & Levacher, V. An efficient organocatalyzed interconversion of silyl ethers to tosylates using DBU and p-toluenesulfonyl fluoride. Synlett 2008, 1463–1466 (2008). An early demonstration of the effectiveness of pairing silicon-activated reagents with sulfonyl fluorides, to achieve efficient reactivity.
Qin, H.-L., Zheng, Q., Bare, G. A. L., Wu, P. & Sharpless, K. B. A Heck–Matsuda process for the synthesis of β-arylethenesulfonyl fluorides: selectively addressable bis-electrophiles for SuFEx click chemistry. Angew. Chem. Int. Ed. 55, 14155–14158 (2016).
Ciuffarin, E., Senatore, L. & Isola, M. Nucleophilic substitution at four-co-ordinate sulphur. Mobility of the leaving group. J. Chem. Soc. Perkin Trans. 2, 468–471 (1972).
Pienta, N. J. & Kessler, R. J. Pentaenyl cations from the photolysis of retinyl acetate. Solvent effects on the leaving group ability and relative nucleophilicities: an unequivocal and quantitative demonstration of the importance of hydrogen bonding. J. Am. Chem. Soc. 114, 2419–2428 (1992).
Zheng, Q. et al. SuFEx-enabled, agnostic discovery of covalent inhibitors of human neutrophil elastase. Proc. Natl Acad. Sci. USA 116, 18808–18814 (2019).
Krutak, J. J., Burpitt, R. D., Moore, W. H. & Hyatt, J. A. Chemistry of ethenesulfonyl fluoride. Fluorosulfonylethylation of organic compounds. J. Org. Chem. 44, 3847–3858 (1979). This report first documented the remarkable reactivity of ESF.
Luy, J.-N. & Tonner, R. Complementary base lowers the barrier in SuFEx click chemistry for primary amine nucleophiles. ACS Omega 5, 31432–31439 (2020).
Wei, M. et al. A broad-spectrum, catalytic amidation of sulfonyl fluorides and fluorosulfates. Angew. Chem. Int. Ed. 60, 7397–7404 (2021).
Barrow, A. S. & Moses, J. E. Synthesis of sulfonyl azides via lewis base activation of sulfonyl fluorides and trimethylsilyl azide. Synlett 27, 1840–1843 (2016).
Smedley, C. J. et al. Bifluoride ion mediated SuFEx trifluoromethylation of sulfonyl fluorides and iminosulfur oxydifluorides. Angew. Chem. Int. Ed. 58, 4552–4556 (2019).
Hyatt, J. A. & White, A. W. Synthesis of aryl alkyl and aryl vinyl sulfones via friedel-crafts reactions of sulfonyl fluorides. Synthesis 1984, 214–217 (1984).
Shirota, Y., Nagai, T. & Tokura, N. The reaction between benzylsulfonyl halide and phenyllithium. Bull. Chem. Soc. Jpn 39, 405–405 (1966).
Frye, L. L., Sullivan, E. L., Cusack, K. P. & Funaro, J. M. Sulfonylation of organometallic reagents with arenesulfonyl fluorides: a simple one-step synthesis of sulfones. J. Org. Chem. 57, 697–701 (1992).
Jang, W. B., Jeon, H. J. & Oh, D. Y. Direct sulfonylation of lithiated alkyl phosphonates with benzenesulfonyl fluoride; facile method for preparation of α-sulfonyl alkyl phosphonates and vinyl sulfones. Synth. Commun. 28, 1253–1256 (1998).
Norris, T. The reaction of arenesulphonyl fluorides with anhydrous aluminium chloride. J. Chem. Soc. Perkin Trans. 1, 1378–1380 (1978).
Lee, C. et al. The emerging applications of sulfur(vi) fluorides in catalysis. ACS Catal. 11, 6578–6589 (2021).
Chinthakindi, P. K., Kruger, H. G., Govender, T., Naicker, T. & Arvidsson, P. I. On-water synthesis of biaryl sulfonyl fluorides. J. Org. Chem. 81, 2618–2623 (2016).
Cherepakha, A. Y. et al. Hetaryl bromides bearing the SO2F group–versatile substrates for palladium-catalyzed C–C coupling reactions. Eur. J. Org. Chem. 6682–6692 (2018).
Fadeyi, O. et al. Chemoselective preparation of clickable aryl sulfonyl fluoride monomers: a toolbox of highly functionalized intermediates for chemical biology probe synthesis. ChemBioChem 17, 1925–1930 (2016). An example of how sulfonyl fluorides can tolerate the multiple reactions needed to prepare a collection of complex chemical probe reagents.
Lou, T. S.-B. & Willis, M. C. Arylsulfonyl fluoride boronic acids: preparation and coupling reactivity. Tetrahedron 76, 130782 (2020).
Hedrick, R. M. Ethylene sulfonyl fluoride and its method of preparation. US Patent US2653973 (1953).
Meng, Y.-P. et al. Ethenesulfonyl fluoride (ESF) and its derivatives in SuFEx click chemistry and more. Synthesis 52, 673–687 (2019).
Chen, J., Huang, B.-q, Wang, Z.-q, Zhang, X.-j & Yan, M. Asymmetric conjugate addition of ethylene sulfonyl fluorides to 3-amido-2-oxindoles: synthesis of chiral spirocyclic oxindole sultams. Org. Lett. 21, 9742–9746 (2019).
Zha, G. F. et al. Palladium-catalyzed fluorosulfonylvinylation of organic iodides. Angew. Chem. Int. Ed. 56, 4849–4852 (2017).
Chinthakindi, P. K. et al. A synthesis of “dual warhead” β-aryl ethenesulfonyl fluorides and one-pot reaction to β-sultams. Org. Lett. 19, 480–483 (2017).
Chen, X.-Y., Wu, Y., Zhou, J., Wang, P. & Yu, J.-Q. Synthesis of β-arylethenesulfonyl fluoride via Pd-catalyzed nondirected C–H alkenylation. Org. Lett. 21, 1426–1429 (2019).
Wang, S.-M., Li, C., Leng, J., Bukhari, S. N. A. & Qin, H.-L. Rhodium(iii)-catalyzed oxidative coupling of N-methoxybenzamides and ethenesulfonyl fluoride: a C–H bond activation strategy for the preparation of 2-aryl ethenesulfonyl fluorides and sulfonyl fluoride substituted γ-lactams. Org. Chem. Front. 5, 1411–1415 (2018).
Wang, S.-M., Moku, B., Leng, J. & Qin, H.-L. Rh-catalyzed carboxylates directed C-H activation for the synthesis of ortho-carboxylic 2-arylethenesulfonyl fluorides: access to unique electrophiles for SuFEx click chemistry. Eur. J. Org. Chem. 4407–4410 (2018).
Ncube, G. & Huestis, M. P. Directed Cp*RhIII-catalyzed fluorosulfonylvinylation of arenes. Organometallics 38, 76–80 (2019).
Chen, H.-R., Hu, Z.-Y., Qin, H.-L. & Tang, H. A novel three-component reaction for constructing indolizine-containing aliphatic sulfonyl fluorides. Org. Chem. Front. 8, 1185–1189 (2021).
Zhang, X., Fang, W.-Y., Lekkala, R., Tang, W. & Qin, H.-L. An easy, general and practical method for the construction of alkyl sulfonyl fluorides. Adv. Synth. Catal. 362, 3358–3363 (2020).
Xu, R., Xu, T., Yang, M., Cao, T. & Liao, S. A rapid access to aliphatic sulfonyl fluorides. Nat. Commun. 10, 3752 (2019).
Zhu, D. Y., Zhang, X. J. & Yan, M. Enantioselective addition of azlactones to ethylene sulfonyl fluoride via dual catalysis. Org. Lett. 23, 4228–4232 (2021). This example shows that catalytic enantioselective additions to ESF are possible.
Chen, J., Zhu, D.-y, Zhang, X.-j & Yan, M. Highly enantioselective addition of N-2,2,2-trifluoroethylisatin ketimines to ethylene sulfonyl fluoride. J. Org. Chem. 86, 3041–3048 (2021).
Ungureanu, A., Levens, A., Candish, L. & Lupton, D. W. N-heterocyclic carbene catalyzed synthesis of delta-sultones via α,β-unsaturated sulfonyl azolium intermediates. Angew. Chem. Int. Ed. 54, 11780–11784 (2015).
Chen, X. et al. Synthesis of a class of fused δ-sultone heterocycles via DBU-catalyzed direct annulative SuFEx click of ethenesulfonyl fluorides and pyrazolones or 1,3-dicarbonyl compounds. Adv. Synth. Catal. 359, 3254–3260 (2017).
Chen, X., Zha, G. F., Fang, W. Y., Rakesh, K. P. & Qin, H. L. A portal to a class of novel sultone-functionalized pyridines via an annulative SuFEx process employing Earth abundant nickel catalysts. Chem. Commun. 54, 9011–9014 (2018).
Khumalo, M. F. et al. Synthesis of novel 1,2,4-thiadiazinane 1,1-dioxides via three component SuFEx type reaction. RSC Adv. 8, 37503–37507 (2018).
Zheng, Q., Dong, J. & Sharpless, K. B. Ethenesulfonyl fluoride (ESF): an on-water procedure for the kilogram-scale preparation. J. Org. Chem. 81, 11360–11362 (2016).
Smedley, C. J. et al. 1-Bromoethene-1-sulfonyl fluoride (BESF) is another good connective hub for SuFEx click chemistry. Chem. Commun. 54, 6020–6023 (2018).
Thomas, J. & Fokin, V. V. Regioselective synthesis of fluorosulfonyl 1,2,3-triazoles from bromovinylsulfonyl fluoride. Org. Lett. 20, 3749–3752 (2018).
Leng, J. & Qin, H.-L. 1-Bromoethene-1-sulfonyl fluoride (1-Br-ESF), a new SuFEx clickable reagent, and its application for regioselective construction of 5-sulfonylfluoro isoxazoles. Chem. Commun. 54, 4477–4480 (2018).
Leng, J., Tang, W., Fang, W. Y., Zhao, C. & Qin, H. L. A simple protocol for the stereoselective construction of enaminyl sulfonyl fluorides. Org. Lett. 22, 4316–4321 (2020).
Li, C., Zheng, Y., Rakesh, K. P. & Qin, H.-L. But-3-ene-1,3-disulfonyl difluoride (BDF): a highly selective SuFEx clickable hub for the quick assembly of sultam-containing aliphatic sulfonyl fluorides. Chem. Commun. 56, 8075–8078 (2020).
Zhang, Z.-W., Wang, S.-M., Fang, W.-Y., Lekkala, R. & Qin, H.-L. Protocol for stereoselective construction of highly functionalized dienyl sulfonyl fluoride warheads. J. Org. Chem. 85, 13721–13734 (2020).
Zhang, X., Moku, B., Leng, J., Rakesh, K. P. & Qin, H.-L. 2-Azidoethane-1-sulfonylfluoride (ASF): a versatile bis-clickable reagent for SuFEx and CuAAC click reactions. Eur. J. Org. Chem. 1763–1769 (2019).
Xu, S. & Cui, S. SuFExable isocyanides for Ugi reaction: synthesis of sulfonyl fluoro peptides. Org. Lett. 23, 5197–5202 (2021).
Li, X.-R. et al. Palladacycle promoted asymmetric hydrophosphination of α,β-unsaturated sulfonyl fluorides. J. Organomet. Chem. 899, 120912 (2019).
Moku, B., Fang, W.-Y., Leng, J., Kantchev, E. A. B. & Qin, H.-L. Rh(i)–diene-catalyzed addition of (hetero)aryl functionality to 1,3-dienylsulfonyl fluorides achieving exclusive regioselectivity and high enantioselectivity: generality and mechanism. ACS Catal. 9, 10477–10488 (2019).
Moku, B. et al. Rh-catalyzed highly enantioselective synthesis of aliphatic sulfonyl fluorides. iScience 21, 695–705 (2019).
Fang, W.-Y., Wang, S.-M., Zhang, Z.-W. & Qin, H.-L. Clickable transformation of nitriles (RCN) to oxazolyl sulfonyl fluoride warheads. Org. Lett. 22, 8904–8909 (2020).
Talko, A., Antoniak, D. & Barbasiewicz, M. Directed ortho-metalation of arenesulfonyl fluorides and aryl fluorosulfates. Synthesis 51, 2278–2286 (2019).
Parker, R. P. & Hofmann, C. M. Sulfonyl fluorides of amino azo dyestuffs. US Patent US2576037 (1947).
Abdul Fattah, T., Saeed, A. & Albericio, F. Recent advances towards sulfur (vi) fluoride exchange (SuFEx) click chemistry. J. Fluor. Chem. 213, 87–112 (2018).
Zhong, T., Chen, Z., Yi, J., Lu, G. & Weng, J. Recent progress in the synthesis of sulfonyl fluorides for SuFEx click chemistry. Chin. Chem. Lett. 32, 2736–2750 (2021).
Niederprüm, H., Voss, P. & Beyl, V. Über Perfluoralkansulfonsäurearylester. Justus Liebigs Ann. Chem. 1973, 20–32 (1973).
Anderson, K. W., Mendez-Perez, M., Priego, J. & Buchwald, S. L. Palladium-catalyzed amination of aryl nonaflates. J. Org. Chem. 68, 9563–9573 (2003).
Barluenga, J., Florentino, L., Aznar, F. & Valdés, C. Synthesis of polysubstituted olefins by Pd-catalyzed cross-coupling reaction of tosylhydrazones and aryl nonaflates. Org. Lett. 13, 510–513 (2011).
Shekhar, S., Dunn, T. B., Kotecki, B. J., Montavon, D. K. & Cullen, S. C. A general method for palladium-catalyzed reactions of primary sulfonamides with aryl nonaflates. J. Org. Chem. 76, 4552–4563 (2011).
Bennua-Skalmowski, B. & Vorbrüggen, H. A facile conversion of primary or secondary alcohols with n-perfluorobutane-sulfonyl fluoride/1,8-diazabicyclo[5.4.0]undec-7-ene into their corresponding fluorides. Tetrahedron Lett. 36, 2611–2614 (1995).
Yin, J., Zarkowsky, D. S., Thomas, D. W., Zhao, M. M. & Huffman, M. A. Direct and convenient conversion of alcohols to fluorides. Org. Lett. 6, 1465–1468 (2004).
Nielsen, M. K., Ahneman, D. T., Riera, O. & Doyle, A. G. Deoxyfluorination with sulfonyl fluorides: navigating reaction space with machine learning. J. Am. Chem. Soc. 140, 5004–5008 (2018).
Nielsen, M. K., Ugaz, C. R., Li, W. & Doyle, A. G. PyFluor: a low-cost, stable, and selective deoxyfluorination reagent. J. Am. Chem. Soc. 137, 9571–9574 (2015). This paper provides an example of a sulfonyl fluoride engineered to act as a fluorinating reagent.
Zhang, C.-P., Chen, Q.-Y., Guo, Y., Xiao, J.-C. & Gu, Y.-C. Difluoromethylation and trifluoromethylation reagents derived from tetrafluoroethane β-sultone: synthesis, reactivity and applications. Coord. Chem. Rev. 261, 28–72 (2014).
Chen, Q. & Zhu, S. Perfluoro-sulfonic and polyfluoro-sulfonic acids. 15. Generation of difluorocarbene and fluorosulfonyldifluoromethide ion from methyl α-fluorosulfonyldifluoroacetate. Sci. Sin. Ser. B 16, 561–568 (1986).
Chen, Q. & Wu, S. Perfluoro- and polyfluorosulfonic acids. 21. Synthesis of difluoromethyl esters using fluorosulfonyldifluoroacetic acid as a difluorocarbene precursor. J. Org. Chem. 54, 3023–3027 (1989).
Tian, F. et al. A novel and highly efficient synthesis of gem-difluorocyclopropanes. Org. Lett. 2, 563–564 (2000).
Jiang, Y., Fang, W.-Y., Rakesh, K. P. & Qin, H.-L. Copper-catalyzed mild desulfonylation of vinyl sulfonyl molecules. Org. Chem. Front. 7, 1696–1702 (2020).
Thomson, D. W. & Ehlers, G. F. L. Aromatic polysulfonates: preparation and properties. J. Polym. Sci. A Gen. Pap. 2, 1051–1056 (1964).
Work, J. L. & Herweh, J. E. Thermal and mechanical properties of some polysulfonates. J. Polym. Sci. A-1 6, 2022–2030 (1968).
Dong, J., Sharpless, K. B., Kwisnek, L., Oakdale, J. S. & Fokin, V. V. SuFEx-based synthesis of polysulfates. Angew. Chem. Int. Ed. 53, 9466–9470 (2014).
Wang, H. et al. SuFEx-based polysulfonate formation from ethenesulfonyl fluoride-amine adducts. Angew. Chem. Int. Ed. 56, 11203–11208 (2017).
Gao, B. et al. Bifluoride-catalysed sulfur(vi) fluoride exchange reaction for the synthesis of polysulfates and polysulfonates. Nat. Chem. 9, 1083–1088 (2017). This paper describes a powerful application of SuFEx reactivity applied to the synthesis of polymers.
Kulow, R. W., Wu, J. W., Kim, C. & Michaudel, Q. Synthesis of unsymmetrical sulfamides and polysulfamides via SuFEx click chemistry. Chem. Sci. 11, 7807–7812 (2020).
Brooks, K. et al. SuFEx postpolymerization modification kinetics and reactivity in polymer brushes. Macromolecules 51, 297–305 (2018).
Yatvin, J., Brooks, K. & Locklin, J. SuFEx on the surface: a flexible platform for postpolymerization modification of polymer brushes. Angew. Chem. Int. Ed. 54, 13370–13373 (2015). This is an impressive example of using SuFEx reactivity to modify polymer surfaces, again exploiting the combination of silyl-activated nucleophiles and sulfonyl fluoride electrophiles.
Brooks, K. et al. Multifunctional surface manipulation using orthogonal click chemistry. Langmuir 32, 6600–6605 (2016).
Gahtory, D. et al. Quantitative and orthogonal formation and reactivity of SuFEx platforms. Chem. Eur. J. 24, 10550–10556 (2018).
Liu, W., Dong, Y., Zhang, S., Wu, Z. & Chen, H. A rapid one-step surface functionalization of polyvinyl chloride by combining click sulfur(vi)-fluoride exchange with benzophenone photochemistry. Chem. Commun. 55, 858–861 (2019).
Dong, Y. et al. “Click-chemical” modification of cellulose acetate nanofibers: a versatile platform for biofunctionalization. J. Mater. Chem. B 6, 4579–4582 (2018).
Park, S. et al. SuFEx in metal–organic frameworks: versatile postsynthetic modification tool. ACS Appl. Mater. Interfaces 10, 33785–33789 (2018).
Liu, S., Cao, Y., Wu, Z. & Chen, H. Reactive films fabricated using click sulfur(vi)–fluoride exchange reactions via layer-by-layer assembly. J. Mater. Chem. B 8, 5529–5534 (2020).
Yatvin, J., Brooks, K. & Locklin, J. SuFEx click: new materials from SOxF and silyl ethers. Chem. Eur. J. 22, 16348–16354 (2016).
Xu, L., Wu, P. & Dong, J. in Synthetic Polymer Chemistry: Innovations and Outlook Ch. 1 (eds Zhao, Z. et al.) 1–31 (Royal Society of Chemistry, 2019).
Shen, C. et al. Stabilizing formamidinium lead iodide perovskite by sulfonyl-functionalized phenethylammonium salt via crystallization control and surface passivation. Sol. RRL 4, 2000069 (2020).
Siegel, D. J. et al. Molecular design principles of ionic liquids with a sulfonyl fluoride moiety. N. J. Chem. 45, 2443–2452 (2021).
Myers, D. K. & Kemp, A. Inhibition of esterases by the fluorides of organic acids. Nature 173, 33–34 (1954).
Gold, A. M. & Fahrney, D. Sulfonyl fluorides as inhibitors of esterases. II. Formation and reactions of phenylmethanesulfonyl α-chymotrypsin. Biochemistry 3, 783–791 (1964).
Gold, A. M. Sulfonyl fluorides as inhibitors of esterases. III. Identification of serine as the site of sulfonylation in phenylmethanesulfonyl α-chymotrypsin. Biochemistry 4, 897–901 (1965).
Baker, B. R. & Lourens, G. J. Irreversible enzyme inhibitors. CV.1,2 Differential irreversible inhibition of vertebrate dihydrofolic reductases by derivatives of 4,6-diamino-1,2-dihydro-2,2-dimethyl-1-phenyl-s-triazines substituted with a terminal sulfonyl fluoride. J. Med. Chem. 10, 1113–1122 (1967).
Jones, L. H. in Annual Reports in Medicinal Chemistry Vol. 56 Ch. 4 (eds Ward, R. A. & Grimster, N. P.) 95–134 (Academic, 2020).
Pal, P. K., Wechter, W. J. & Colman, R. F. Affinity labeling of the inhibitory DPNH site of bovine liver glutamate dehydrogenase by 5′-fluorosulfonylbenzoyl adenosine. J. Biol. Chem. 250, 8140–8147 (1975).
Colman, R. F. Affinity labeling of purine nucleotide sites in proteins. Annu. Rev. Biochem. 52, 67–91 (1983).
Parker, C. G. & Pratt, M. R. Click chemistry in proteomic investigations. Cell 180, 605–632 (2020).
Gu, C. et al. Chemical proteomics with sulfonyl fluoride probes reveals selective labeling of functional tyrosines in glutathione transferases. Chem. Biol. 20, 541–548 (2013).
Zhao, Q. et al. Broad-spectrum kinase profiling in live cells with lysine-targeted sulfonyl fluoride probes. J. Am. Chem. Soc. 139, 680–685 (2017). An impressive application of multifunctional sulfonyl fluoride reagents to target specific lysine residues.
Yan, X. et al. Europium-labeled activity-based probe through click chemistry: absolute serine protease quantification using 153Eu isotope dilution ICP/MS. Angew. Chem. Int. Ed. 51, 3358–3363 (2012).
Jones, L. H. & Kelly, J. W. Structure-based design and analysis of SuFEx chemical probes. RSC Med. Chem. 11, 10–17 (2020).
Zelli, R., Tommasone, S., Dumy, P., Marra, A. & Dondoni, A. A click ligation based on SuFEx for the metal-free synthesis of sugar and iminosugar clusters. Eur. J. Org. Chem. 5102–5116 (2016).
Yang, B. et al. Proximity-enhanced SuFEx chemical cross-linker for specific and multitargeting cross-linking mass spectrometry. Proc. Natl Acad. Sci. USA 115, 11162–11167 (2018).
Miller, P. W., Long, N. J., Vilar, R. & Gee, A. D. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew. Chem. Int. Ed. 47, 8998–9033 (2008).
Schirrmacher, R. et al. Small prosthetic groups in 18F-radiochemistry: useful auxiliaries for the design of 18F-PET tracers. Semin. Nucl. Med. 47, 474–492 (2017).
Inkster, J. A. H. et al. Sulfonyl fluoride-based prosthetic compounds as potential 18F labelling agents. Chem. Eur. J. 18, 11079–11087 (2012).
Matesic, L. et al. Ascertaining the suitability of aryl sulfonyl fluorides for [18F]radiochemistry applications: a systematic investigation using microfluidics. J. Org. Chem. 78, 11262–11270 (2013).
Wang, J. & van Dam, R. M. High-efficiency production of radiopharmaceuticals via droplet radiochemistry: a review of recent progress. Mol. Imaging 19, 1536012120973099 (2020).
Zhou, D. et al. Preliminary evaluation of a novel 18F-labeled PARP-1 ligand for PET imaging of PARP-1 expression in prostate cancer. Nucl. Med. Biol. 66, 26–31 (2018).
Eby, R. & Schuerch, C. The use of 1-O-tosyl-d-glucopyranose derivatives in α-d-glucoside synthesis. Carbohydr. Res. 34, 79–90 (1974).
Ametamey, S. M., Honer, M. & Schubiger, P. A. Molecular imaging with PET. Chem. Rev. 108, 1501–1516 (2008).
Zhang, B. et al. Synthesis, bioconjugation and stability studies of [18F]ethenesulfonyl fluoride. J. Label. Compd. Radiopharm. 61, 847–856 (2018). This shows how ESF, an important reagent for the introduction of sulfonyl fluorides, can be adapted to 18F-labelling.
Zhang, B. et al. [18F]ethenesulfonyl fluoride as a practical radiofluoride relay reagent. Chem. Eur. J. 25, 7613–7617 (2019).
Pascali, G. et al. Sulfur–fluorine bond in PET radiochemistry. EJNMMI Radiopharm. Chem. 2, 9 (2017).
Montanino, M., Passerini, S. & Appetecchi, G. B. in Rechargeable Lithium Batteries (ed. Franco, A.) 73–116 (Woodhead, 2015).
Li, Q., Chen, J., Fan, L., Kong, X. & Lu, Y. Progress in electrolytes for rechargeable Li-based batteries and beyond. Green Energy Environ. 1, 18–42 (2016).
Razzini, G., Rovellini, S., Alessandrini, F., Di Pietro, B. & Scrosati, B. The lithium-sulfuryl chloride battery: discharge behaviour. J. Power Sources 5, 263–271 (1980).
Fu, X. et al. Sulfuryl chloride as a functional additive towards dendrite-free and long-life Li metal anodes. J. Mater. Chem. A 7, 25003–25009 (2019).
Lee, S.-Y., Ueno, K. & Angell, C. A. Lithium salt solutions in mixed sulfone and sulfone-carbonate solvents: a Walden plot analysis of the maximally conductive compositions. J. Phys. Chem. C. 116, 23915–23920 (2012).
Xue, L., Ueno, K., Lee, S.-Y. & Angell, C. A. Enhanced performance of sulfone-based electrolytes at lithium ion battery electrodes, including the LiNi0.5Mn1.5O4 high voltage cathode. J. Power Sources 262, 123–128 (2014).
Che, Y. et al. Protective electrode/electrolyte interphases for high energy lithium-ion batteries with p-toluenesulfonyl fluoride electrolyte additive. J. Energy Chem. 52, 361–371 (2021).
Xue, W. et al. FSI-inspired solvent and “full fluorosulfonyl” electrolyte for 4V class lithium-metal batteries. Energy Environ. Sci. 13, 212–220 (2020).
Conte, L., Gambaretto, G., Caporiccio, G., Alessandrini, F. & Passerini, S. Perfluoroalkanesulfonylimides and their lithium salts: synthesis and characterisation of intermediates and target compounds. J. Fluor. Chem. 125, 243–252 (2004).
Jin, Z., Xie, K. & Hong, X. Electrochemical performance of lithium/sulfur batteries using perfluorinated ionomer electrolyte with lithium sulfonyl dicyanomethide functional groups as functional separator. RSC Adv. 3, 8889–8898 (2013).
Zhou, H. et al. Introduction of a crystalline, shelf-stable reagent for the synthesis of sulfur(vi) fluorides. Org. Lett. 20, 812–815 (2018).
Guo, T. et al. A new portal to SuFEx click chemistry: a stable fluorosulfuryl imidazolium salt emerging as an “F−SO2+” donor of unprecedented reactivity, selectivity, and scope. Angew. Chem. Int. Ed. 57, 2605–2610 (2018).
Börgel, J. & Ritter, T. Late-stage functionalization. Chem 6, 1877–1887 (2020).
Brzozowski, M., O’Brien, M., Ley, S. V. & Polyzos, A. Flow chemistry: intelligent processing of gas–liquid transformations using a tube-in-tube reactor. Acc. Chem. Res. 48, 349–362 (2015).
Malet-Sanz, L., Madrzak, J., Ley, S. V. & Baxendale, I. R. Preparation of arylsulfonyl chlorides by chlorosulfonylation of in situ generated diazonium salts using a continuous flow reactor. Org. Biomol. Chem. 8, 5324–5332 (2010).
Liu, Z. et al. SuFEx click chemistry enabled late-stage drug functionalization. J. Am. Chem. Soc. 140, 2919–2925 (2018).
Mortenson, D. E. et al. “Inverse drug discovery” strategy to identify proteins that are targeted by latent electrophiles as exemplified by aryl fluorosulfates. J. Am. Chem. Soc. 140, 200–210 (2018).
Wang, N. et al. genetically encoding fluorosulfate-l-tyrosine to react with lysine, histidine, and tyrosine via SuFEx in proteins in vivo. J. Am. Chem. Soc. 140, 4995–4999 (2018).
Martín-Gago, P. & Olsen, C. A. Arylfluorosulfate-based electrophiles for covalent protein labeling: a new addition to the arsenal. Angew. Chem. Int. Ed. 58, 957–966 (2019).
Kassick, A. J. et al. SuFEx-based strategies for the preparation of functional particles and cation exchange resins. Chem. Commun. 55, 3891–3894 (2019).
Xu, H. et al. DNA-encoded libraries: aryl fluorosulfonates as versatile electrophiles enabling facile on-DNA Suzuki, Sonogashira, and Buchwald reactions. Adv. Sci. 6, 1901551 (2019).
Zheng, Q. et al. Sulfur [18F]fluoride exchange click chemistry enabled ultrafast late-stage radiosynthesis. J. Am. Chem. Soc. 143, 3753–3763 (2021).
Li, S., Wu, P., Moses, J. E. & Sharpless, K. B. Multidimensional SuFEx click chemistry: sequential sulfur(vi) fluoride exchange connections of diverse modules launched from an SOF4 hub. Angew. Chem. Int. Ed. 56, 2903–2908 (2017). An impressive demonstration of how simple nucleophiles can be combined using multi-reactive electrophilic S-F hubs to construct a range of S(vi)-functional groups.
Liu, F. et al. Biocompatible SuFEx click chemistry: thionyl tetrafluoride (SOF4)-derived connective hubs for bioconjugation to DNA and proteins. Angew. Chem. Int. Ed. 58, 8029–8033 (2019).
Kitamura, S. et al. Sulfur(vi) fluoride exchange (SuFEx)-enabled high-throughput medicinal chemistry. J. Am. Chem. Soc. 142, 10899–10904 (2020).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Chemistry thanks H.-L. Qin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Bactericidal
-
Having the ability to kill bacteria, as compared to ‘bacteriostatic’ which inhibits bacterial growth or reproduction.
- LD50
-
Median lethal dose, the dose required to kill half of the sample population, expressed as the mass of chemical administered per body mass of test subject.
- Click reactions
-
Thermodynamically favoured processes of joining modular building blocks, which are highly selective, high-yielding, operationally simple and insensitive to oxygen and water.
- Ionic liquids
-
Salts that are liquid at temperatures below 100 °C.
- Activity-based protein probes
-
Small molecules composed of a functional group capable of binding with target proteins, and a second reactive site (reporter) for protein analysis or transformation.
- Positron emission tomography
-
(PET). An imaging technique used in nuclear medicine that measures the metabolic activity of the cells of body tissues, through the administration of a radiotracer.
- Passivation
-
Formation of a coating on a material that is more inert than the material itself.
- Ionomer
-
A polymer that consists of electrically neutral repeating units and a fraction of ionized units distributed along the polymer backbone.
Rights and permissions
About this article
Cite this article
Lou, T.SB., Willis, M.C. Sulfonyl fluorides as targets and substrates in the development of new synthetic methods. Nat Rev Chem 6, 146–162 (2022). https://doi.org/10.1038/s41570-021-00352-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-021-00352-8
This article is cited by
-
Sulfonate derivatives bearing an amide unit: design, synthesis and biological activity studies
BMC Chemistry (2024)
-
C-SuFEx linkage of sulfonimidoyl fluorides and organotrifluoroborates
Nature Communications (2024)
-
Enantioselective sulfur(VI) fluoride exchange reaction of iminosulfur oxydifluorides
Nature Chemistry (2024)
-
Iterative SuFEx approach for sequence-regulated oligosulfates and its extension to periodic copolymers
Nature Communications (2024)
-
Electroreductive hydroxy fluorosulfonylation of alkenes
Nature Communications (2023)