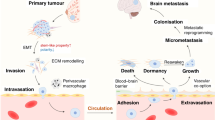
Abstract
Brain metastasis, which commonly arises in patients with lung cancer, breast cancer and melanoma, is associated with poor survival outcomes and poses distinct clinical challenges. The brain microenvironment, with its unique cell types, anatomical structures, metabolic constraints and immune environment, differs drastically from microenvironments of extracranial lesions, imposing a distinct and profound selective pressure on tumour cells that, in turn, shapes the metastatic process and therapeutic responses. Accordingly, the study of brain metastasis could uncover new therapeutic targets and identify novel treatment approaches to address the unmet clinical need. Moreover, such efforts could provide insight into the biology of primary brain tumours, which face similar challenges to brain metastases of extracranial origin, and vice versa. However, the paucity of robust preclinical models of brain metastasis has severely limited such investigations, underscoring the importance of developing improved experimental models that holistically encompass the metastatic cascade and/or brain microenvironment. In this Viewpoint, we asked four leading experts to provide their opinions on these important aspects of brain metastasis biology and management.
The contributors
Adrienne Boire is an assistant attending neurologist and assistant member of the Human Oncology and Pathogenesis Program at Memorial Sloan Kettering Cancer Center, New York, USA. Her clinical and basic science research focuses on leptomeningeal metastases. Her laboratory research programme focuses on microenvironmental interactions between cancer cells and the leptomeninges, and her clinical practice and research focuses on the care of patients with leptomeningeal metastasis.
Priscilla Brastianos is the Director of the Central Nervous System Metastasis Center at Massachusetts General Hospital and Harvard Medical School, Boston, USA. Her research focuses on understanding the molecular mechanisms that drive brain metastasis. Her work demonstrates that brain metastases show branched or divergent evolution, and harbour clinically significant drivers that are distinct from clinically sampled primary tumours. She has translated her scientific findings to national multicentre trials. She also leads a multidisciplinary central nervous system metastasis clinic at Massachusetts General Hospital and Harvard Medical School.
Livia Garzia is an assistant professor with the Department of Surgery, Division of Orthopaedic Surgery at McGill University and a principal investigator at the Research Institute of the McGill University Health Centre, in Montreal, Canada. The main focus of her laboratory is to understand the mechanisms of metastasis and relapse in paediatric solid tumours such as sarcomas and brain tumours. Her research has uncovered the role of haematogenous dissemination of medulloblastoma tumour cells to leptomeningeal metastasis.
Manuel Valiente is the Head of the Brain Metastasis Group at the National Cancer Research Center (CNIO) in Madrid, Spain. His main interest is to study the mechanisms used by metastatic cells to adapt to the brain and to understand how this process modifies both cancer cells and brain cells. His research has proved that the underlying molecular regulation of organ adaptation can be exploited therapeutically using novel strategies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Valiente, M. et al. The evolving landscape of brain metastasis. Trends Cancer 4, 176–196 (2018).
Boire, A. et al. Complement component 3 adapts the cerebrospinal fluid for leptomeningeal metastasis. Cell 168, 1101–1113 (2017).
Neman, J. et al. Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proc. Natl Acad. Sci. USA 111, 984–989 (2014).
Berghoff, A. S. et al. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology 5, e1057388 (2016).
Sevenich, L. et al. Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat. Cell Biol. 16, 876–888 (2014).
Tawbi, H. A. et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N. Engl. J. Med. 379, 722–730 (2018).
Long, G. V. et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 19, 672–681 (2018).
Lun, M. P., Monuki, E. S. & Lehtinen, M. K. Development and functions of the choroid plexus–cerebrospinal fluid system. Nat. Rev. Neurosci. 16, 445–457 (2015).
Banks, W. A. From blood–brain barrier to blood–brain interface: new opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 15, 275–292 (2016).
Follain, G. et al. Hemodynamic forces tune the arrest, adhesion, and extravasation of circulating tumor cells. Dev. Cell 45, 33–52 (2018).
Kienast, Y. et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat. Med. 16, 116–122 (2010).
Lorger, M. & Felding-Habermann, B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am. J. Pathol. 176, 2958–2971 (2010).
Valiente, M. et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 156, 1002–1016 (2014).
Zhang, L. et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 527, 100–104 (2015).
Priego, N. et al. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis article. Nat. Med. 24, 1481 (2018).
da Fonseca, A. C. C. et al. The impact of microglial activation on blood–brain barrier in brain diseases. Front. Cell. Neurosci. 8, 362 (2014).
Seano, G. et al. Solid stress in brain tumours causes neuronal loss and neurological dysfunction and can be reversed by lithium. Nat. Biomed. Eng. 3, 230–245 (2019).
Venkatesh, H. S. et al. Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell 161, 803–816 (2015).
Venkatesh, H. S. et al. Electrical and synaptic integration of glioma into neural circuits. Nature 573, 539–545 (2019).
Zeng, Q. et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 573, 526–531 (2019).
Yoo, B. C. et al. Cerebrospinal fluid metabolomic profiles can discriminate patients with leptomeningeal carcinomatosis from patients at high risk for leptomeningeal metastasis. Oncotarget 8, 101203–101214 (2017).
Gholamin, S. et al. Disrupting the CD47–SIRPα anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci. Transl Med. 9, eaaf2968 (2017).
Zhang, X. H.-F. et al. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell 154, 1060–1073 (2013).
Louie, E. et al. Neurotrophin-3 modulates breast cancer cells and the microenvironment to promote the growth of breast cancer brain metastasis. Oncogene 32, 4064–4077 (2013).
Chen, Q. et al. Carcinoma–astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 533, 493–498 (2016).
Venkataramani, V. et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 573, 532–538 (2019).
Lockman, P. R. et al. Heterogeneous blood–tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin. Cancer Res. 16, 5664–5678 (2010).
Lyle, L. T. et al. Alterations in pericyte subpopulations are associated with elevated blood–tumor barrier permeability in experimental brain metastasis of breast cancer. Clin. Cancer Res. 22, 5287–5299 (2016).
Griveau, A. et al. A glial signature and Wnt7 signaling regulate glioma–vascular interactions and tumor microenvironment. Cancer Cell 33, 874–889.e7 (2018).
Brastianos, P. K. et al. Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat. Genet. 46, 161–165 (2014).
Juratli, T. A. et al. Targeted treatment of papillary craniopharyngiomas harboring BRAF V600E mutations. Cancer 125, 2910–2914 (2019).
Drilon, A. et al. Safety and antitumor activity of the multitargeted Pan-TRK, ROS1, and ALK inhibitor entrectinib: combined results from two phase I trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 7, 400–409 (2017).
Jacob, L. S. et al. Metastatic competence can emerge with selection of preexisting oncogenic alleles without a need of new mutations. Cancer Res. 75, 3713–3719 (2015).
Greaves, M. & Maley, C. C. Clonal evolution in cancer. Nature 481, 306–313 (2012).
Shah, S. P. et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 486, 395–399 (2012).
Bowman, R. L. et al. Macrophage ontogeny underlies differences in tumor-specific education in brain malignancies. Cell Rep. 17, 2445–2459 (2016).
Seute, T., Leffers, P., Wilmink, J. T., Ten Velde, G. P. M. & Twijnstra, A. Response of asymptomatic brain metastases from small-cell lung cancer to systemic first-line chemotherapy. J. Clin. Oncol. 24, 2079–2083 (2006).
Qian, B. Z. et al. CCL2 recruits inflammatory monocytes to facilitate breast–tumour metastasis. Nature 475, 222–475 (2011).
Garzia, L. et al. A hematogenous route for medulloblastoma leptomeningeal metastases. Cell 172, 1050–1062.e14 (2018).
Taggart, D. et al. Anti-PD-1/anti-CTLA-4 efficacy in melanoma brain metastases depends on extracranial disease and augmentation of CD8+ T cell trafficking. Proc. Natl Acad. Sci. USA 115, E1540–E1549 (2018).
Cao, K. I. et al. Phase II randomized study of whole-brain radiation therapy with or without concurrent temozolomide for brain metastases from breast cancer. Ann. Oncol. 26, 89–94 (2015).
Palmieri, D. et al. Profound prevention of experimental brain metastases of breast cancer by temozolomide in an MGMT-dependent manner. Clin. Cancer Res. 20, 2727–2739 (2014).
Sunwoo, L. et al. Differentiation of glioblastoma from brain metastasis: qualitative and quantitative analysis using arterial spin labeling MR imaging. PLOS ONE 11, e0166662 (2016).
Kuczynski, E. A., Vermeulen, P. B., Pezzella, F., Kerbel, R. S. & Reynolds, A. R. Vessel co-option in cancer. Nat. Rev. Clin. Oncol. 16, 469–493 (2019).
Mashimo, T. et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell 159, 1603–1614 (2014).
Chen, J. et al. Gain of glucose-independent growth upon metastasis of breast cancer cells to the brain. Cancer Res. 75, 554–565 (2015).
Bos, P. D. et al. Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1009 (2009).
Ippen, F. M. et al. The dual PI3K/mTOR pathway inhibitor GDC-0084 achieves antitumor activity in PIK3CA-mutant breast cancer brain metastases. Clin. Cancer Res. 25, 3374–3383 (2019).
Meuwissen, R. et al. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell 4, 181–189 (2003).
Kato, M. et al. Transgenic mouse model for skin malignant melanoma. Oncogene 17, 1885–1888 (1998).
Cho, J. H. et al. AKT1 activation promotes development of melanoma metastases. Cell Rep. 13, 898–905 (2015).
Wu, X. et al. Clonal selection drives genetic divergence of metastatic medulloblastoma. Nature 482, 529–533 (2012).
Moriarity, B. S. et al. A Sleeping Beauty forward genetic screen identifies new genes and pathways driving osteosarcoma development and metastasis. Nat. Genet. 47, 615–624 (2015).
De La Rochere, P. et al. Humanized mice for the study of immuno-oncology. Trends Immunol. 39, 748–763 (2018).
Winslow, M. M. et al. Suppression of lung adenocarcinoma progression by Nkx2-1. Nature 473, 101–104 (2011).
Schade, B. et al. PTEN deficiency in a luminal ErbB-2 mouse model results in dramatic acceleration of mammary tumorigenesis and metastasis. J. Biol. Chem. 284, 19018–19026 (2009).
Kircher, D. A. et al. AKT1E17K activates focal adhesion kinase and promotes melanoma brain metastasis. Mol. Cancer Res. 17, 1787–1800 (2019).
Fischer, G. M. et al. Molecular profiling reveals unique immune and metabolic features of melanoma brain metastases. Cancer Discov. 9, 628–645 (2019).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Louveau, A. et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 21, 1380–1391 (2018).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl Med. 4, 147ra111 (2012).
Lin, N. U. et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 16, e270–e278 (2015).
Timmer, M. et al. Discordance and conversion rates of progesterone-, estrogen-, and HER2/neu-receptor status in primary breast cancer and brain metastasis mainly triggered by hormone therapy. Anticancer Res. 37, 4859–4865 (2017).
Priedigkeit, N. et al. Intrinsic subtype switching and acquired ERBB2/HER2 amplifications and mutations in breast cancer brain metastases. JAMA Oncol. 3, 666–671 (2017).
Brastianos, P. K. et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 5, 1164–1177 (2015).
Nygaard, V., Prasmickaite, L., Vasiliauskaite, K., Clancy, T. & Hovig, E. Melanoma brain colonization involves the emergence of a brain-adaptive phenotype. Oncoscience 1, 82–94 (2014).
Zaugg, K. et al. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes Dev. 25, 1041–1051 (2011).
Venning, F. A., Wullkopf, L. & Erler, J. T. Targeting ECM disrupts cancer progression. Front. Oncol. 5, 224 (2015).
Park, E. S. et al. Cross-species hybridization of microarrays for studying tumor transcriptome of brain metastasis. Proc. Natl Acad. Sci. USA 108, 17456–17461 (2011).
Acknowledgements
M.V. acknowledges support from the Ministry of Economy and Competitiveness (MINECO) grant MINECO-Retos SAF2017-89643-R, the Bristol-Myers Squibb–Melanoma Research Alliance Young Investigator Award 2017 (498103), the Beug Foundation’s Prize for Metastasis Research 2017, Fundación Ramón Areces (CIVP19S8163), Worldwide Cancer Research (19-0177), Horizon 2020 Funding and Emerging Technologies (FET) Open (828972), the Clinic and Laboratory Integration Program Cancer Research Institute (CRI) Award 2018 (54545) and Spanish Association Against Cancer (AECC) Coordinated Translational Groups 2017 (GCTRA16015SEOA). M.V. is a Ramón y Cajal Investigator (RYC-2013-13365) and a member of the European Molecular Biology Organization Young Investigator Programme (EMBO YIP; 4053). L.G. is supported by CIHR Operating Grant PJT-162234, Terry Fox Research Institute grant TFRI1087, Canadian Cancer Society Research Institute CCS#705799, the Cancer Research Society and the C17 Research Network.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
A.B. is a Consultant for Arix Biosciences, and has patents related to methods for treating brain metastasis (US Patent 10,413,522) and modulating permeability of the blood–CSF barrier (US Provisional Application Number 62/258,044). P.B. has consulted for Lilly, Genentech–Roche, AngioChem and Tesaro, has received speakers honoraria from Merck and Genentech–Roche, and has received research funding from Merck, Bristol-Myers Squibb and Pfizer. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Boire, A., Brastianos, P.K., Garzia, L. et al. Brain metastasis. Nat Rev Cancer 20, 4–11 (2020). https://doi.org/10.1038/s41568-019-0220-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-019-0220-y
This article is cited by
-
Anti-EGFR ScFv functionalized exosomes delivering LPCAT1 specific siRNAs for inhibition of lung cancer brain metastases
Journal of Nanobiotechnology (2024)
-
CNN-based multi-modal radiomics analysis of pseudo-CT utilization in MRI-only brain stereotactic radiotherapy: a feasibility study
BMC Cancer (2024)
-
A large open access dataset of brain metastasis 3D segmentations on MRI with clinical and imaging information
Scientific Data (2024)
-
Investigation of GPR143 as a promising novel marker for the progression of skin cutaneous melanoma through bioinformatic analyses and cell experiments
Apoptosis (2024)
-
From pre-clinical to translational brain metastasis research: current challenges and emerging opportunities
Clinical & Experimental Metastasis (2024)