Abstract
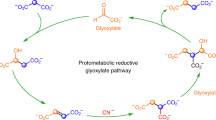
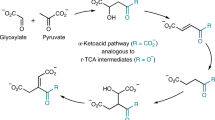
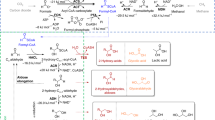
The reverse tricarboxylic acid cycle (rTCA) is a central anabolic network that uses carbon dioxide (CO2) and may have provided complex carbon substrates for life before the advent of RNA or enzymes. However, non-enzymatic promotion of the rTCA cycle, in particular carbon fixation, remains challenging, even with primordial metal catalysis. Here, we report that the fixation of CO2 by reductive carboxylation of succinate and α-ketoglutarate was achieved in aqueous microdroplets under ambient conditions without the use of catalysts. Under identical conditions, the aqueous microdroplets also facilitated the sequences in the rTCA cycle, including reduction, hydration, dehydration and retro-aldol cleavage and linked with the glyoxylate cycle. These reactions of the rTCA cycle were compatible with the aqueous microdroplets, as demonstrated with two-reaction and four-reaction sequences. A higher selectivity giving higher product yields was also observed. Our results suggest that the microdroplets provide an energetically favourable microenvironment and facilitate a non-enzymatic version of the rTCA cycle in prebiotic carbon anabolism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All the results supporting the findings are in Supplementary Information. Source data are provided with this paper.
References
Muchowska, K. B., Varma, S. J. & Moran, J. Nonenzymatic metabolic reactions and life’s origins. Chem. Rev. 120, 7708–7744 (2020).
Berg, I. A. et al. Autotrophic carbon fixation in Archaea. Nat. Rev. Microbiol. 8, 447–460 (2010).
Preiner, M. et al. A hydrogen-dependent geochemical analogue of primordial carbon and energy metabolism. Nat. Ecol. Evol. 4, 534–542 (2020).
Ianeselli, A. et al. Water cycles in a Hadean CO2 atmosphere drive the evolution of long DNA. Nat. Phys. 18, 579–585 (2022).
Hudson, R. et al. CO2 reduction driven by a pH gradient. Proc. Natl Acad. Sci. USA 117, 22873–22879 (2020).
Gibard, C., Bhowmik, S., Karki, M., Kim, E. K. & Krishnamurthy, R. Phosphorylation, oligomerization and self-assembly in water under potential prebiotic conditions. Nat. Chem. 10, 212–217 (2018).
Ruiz-Mirazo, K., Briones, C. & de la Escosura, A. Prebiotic systems chemistry: new perspectives for the origins of life. Chem. Rev. 114, 285–366 (2014).
Kitadai, N. et al. Metals likely promoted protometabolism in early ocean alkaline hydrothermal systems. Sci. Adv. 5, eaav7848 (2019).
Novikov, Y. & Copley, S. D. Reactivity landscape of pyruvate under simulated hydrothermal vent conditions. Proc. Natl Acad. Sci. USA 110, 13283–13288 (2013).
Martin, W. & Russell, M. J. On the origin of biochemistry at an alkaline hydrothermal vent. Phil. Trans. R. Soc. B 362, 1887–1925 (2007).
Wachtershauser, G. Before enzymes and templaters—theory of surface metabolism. Microbiol. Rev. 52, 452–484 (1988).
Varma, S. J., Muchowska, K. B., Chatelain, P. & Moran, J. Native iron reduces CO2 to intermediates and end-products of the acetyl-CoA pathway. Nat. Ecol. Evol. 2, 1019–1024 (2018).
Cody, G. D. et al. Primordial carbonylated iron–sulfur compounds and the synthesis of pyruvate. Science 289, 1337–1339 (2000).
Keller, M. A., Kampjut, D., Harrison, S. A. & Ralser, M. Sulfate radicals enable a non-enzymatic Krebs cycle precursor. Nat. Ecol. Evol. 1, 0083 (2017).
Smith, E. & Morowitz, H. J. Universality in intermediary metabolism. Proc. Natl Acad. Sci. USA 101, 13168–13173 (2004).
Evans, M. C., Buchanan, B. B. & Arnon, D. I. A new ferredoxin-dependent carbon reduction cycle in a photosynthetic bacterium. Proc. Natl Acad. Sci. USA 55, 928–934 (1966).
Srinivasan, V. & Morowitz, H. J. Analysis of the intermediary metabolism of a reductive chemoautotroph. Biol. Bull. 217, 222–232 (2009).
Morowitz, H. J., Kostelnik, J. D., Yang, J. & Cody, G. D. The origin of intermediary metabolism. Proc. Natl Acad. Sci. USA 97, 7704–7708 (2000).
Wachtershauser, G. Evolution of the 1st metabolic cycles. Proc. Natl Acad. Sci. USA 87, 200–204 (1990).
Carbonell, P., Lecointre, G. & Faulon, J. L. Origins of specificity and promiscuity in metabolic networks. J. Biol. Chem. 286, 43994–44004 (2011).
Kitadai, N., Kameya, M. & Fujishima, K. Origin of the reductive tricarboxylic acid (rTCA) cycle-type CO2 fixation: a perspective. Life 7, 39–44 (2017).
Codya, G. D. et al. Geochemical roots of autotrophic carbon fixation: hydrothermal experiments in the system citric acid, H2O-(±FeS)-(±NiS). Geochim. Cosmochim. Acta 65, 3557–3576 (2001).
Muchowska, K. B. et al. Metals promote sequences of the reverse Krebs cycle. Nat. Ecol. Evol. 1, 1716–1721 (2017).
Zhang, X. V. & Martin, S. T. Driving parts of Krebs cycle in reverse through mineral photochemistry. J. Am. Chem. Soc. 128, 16032–16033 (2006).
Muchowska, K. B., Varma, S. J. & Moran, J. Synthesis and breakdown of universal metabolic precursors promoted by iron. Nature 569, 104–107 (2019).
Rauscher, S. A. & Moran, J. Hydrogen drives part of the reverse Krebs cycle under metal or meteorite catalysis. Angew. Chem. Int. Ed. Engl. 61, e202212932 (2022).
Springsteen, G., Yerabolu, J. R., Nelson, J., Rhea, C. J. & Krishnamurthy, R. Linked cycles of oxidative decarboxylation of glyoxylate as protometabolic analogs of the citric acid cycle. Nat. Commun. 9, 91–99 (2018).
Ju, Y. et al. Aqueous-microdroplet-driven abiotic synthesis of ribonucleotides. J. Phys. Chem. Lett. 13, 567–573 (2022).
Nam, I., Lee, J. K., Nam, H. G. & Zare, R. N. Abiotic production of sugar phosphates and uridine ribonucleoside in aqueous microdroplets. Proc. Natl Acad. Sci. USA 114, 12396–12400 (2017).
Lee, J. K., Samanta, D., Nam, H. G. & Zare, R. N. Micrometer-sized water droplets induce spontaneous reduction. J. Am. Chem. Soc. 141, 10585–10589 (2019).
Wei, Z., Li, Y., Cooks, R. G. & Yan, X. Accelerated reaction kinetics in microdroplets: overview and recent developments. Annu. Rev. Phys. Chem. 71, 31–51 (2020).
Zhao, L. et al. Sprayed water microdroplets containing dissolved pyridine spontaneously generate pyridyl anions. Proc. Natl Acad. Sci. USA 119, e2200991119 (2022).
Ju, Y. et al. Abiotic synthesis with plausible emergence for primitive phospholipid in aqueous microdroplets. J. Colloid Interface Sci. 634, 535–542 (2023).
Wang, W. et al. Water microdroplets allow spontaneously abiotic production of peptides. J. Phys. Chem. Lett. 12, 5774–5780 (2021).
Teunissen, S. F., Fernandes, A. M. A. P., Eberlin, M. N. & Alberici, R. M. Celebrating 10 years of easy ambient sonic-spray ionization. TrAC-Trend Anal. Chem. 90, 135–141 (2017).
Venter, A., Sojka, P. E. & Cooks, R. G. Droplet dynamics and ionization mechanisms in desorption electrospray ionization mass spectrometry. Anal. Chem. 78, 8549–8555 (2006).
Smith, J. N., Flagan, R. C. & Beauchamp, J. L. Droplet evaporation and discharge dynamics in electrospray ionization. J. Phys. Chem. A 106, 9957–9967 (2002).
Ganan-Calvo, A. M., Davila, J. & Barrero, A. Current and droplet size in the electrospraying of liquids: scaling laws. J. Aerosol Sci. 28, 249–275 (1997).
Gong, C. et al. Spontaneous reduction-induced degradation of viologen compounds in water microdroplets and its inhibition by host–guest complexation. J. Am. Chem. Soc. 144, 3510–3516 (2022).
Song, X., Meng, Y. & Zare, R. N. Spraying water microdroplets containing 1,2,3-triazole converts carbon dioxide into formic acid. J. Am. Chem. Soc. 144, 16744–16748 (2022).
Huang, K.-H., Wei, Z. & Cooks, R. G. Accelerated reactions of amines with carbon dioxide driven by superacid at the microdroplet interface. Chem. Sci. 12, 2242–2250 (2021).
Feng, L. et al. Ammonium bicarbonate significantly accelerates the microdroplet reactions of amines with carbon dioxide. Anal. Chem. 93, 15775–15784 (2021).
Nam, I., Nam, H. G. & Zare, R. N. Abiotic synthesis of purine and pyrimidine ribonucleosides in aqueous microdroplets. Proc. Natl Acad. Sci. USA 115, 36–40 (2018).
Yan, X., Bain, R. M. & Cooks, R. G. Organic reactions in microdroplets: reaction acceleration revealed by mass spectrometry. Angew. Chem. Int. Ed. 55, 12960–12972 (2016).
Li, Y. et al. Accelerated forced degradation of pharmaceuticals in levitated microdroplet reactors. Chemistry 23, 7349–7353 (2018).
Chen, H., Eberlin, L. S. & Cooks, R. G. Neutral fragment mass spectra via ambient thermal dissociation of peptide and protein ions. J. Am. Chem. Soc. 129, 5880–5886 (2007).
Lee, J. K., Kim, S., Nam, H. G. & Zare, R. N. Microdroplet fusion mass spectrometry for fast reaction kinetics. Proc. Natl Acad. Sci. USA 112, 3898–3903 (2015).
Lhee, S. et al. Spatial localization of charged molecules by salt ions in oil-confined water microdroplets. Sci. Adv. 6, eaba01811 (2020).
Zhang, D., Yuan, X., Gong, C. & Zhang, X. High electric field on water microdroplets catalyzes spontaneous and ultrafast oxidative C-H/N-H cross-coupling. J. Am. Chem. Soc. 144, 16184–16190 (2022).
Kathmann, S. M., Kuo, I. & Mundy, C. J. Electronic effects on the surface potential at the vapor–liquid interface of water. J. Am. Chem. Soc. 130, 16556–16561 (2008).
Qiu, L. & Cooks, R. G. Simultaneous and spontaneous oxidation and reduction in microdroplets by the water radical cation/anion pair. Angew. Chem. Int. Ed. 61, e202210765 (2022).
Wei, H. et al. Aerosol microdroplets exhibit a stable pH gradient. Proc. Natl Acad. Sci. USA 115, 7272–7277 (2018).
Narendra, N. et al. Quantum mechanical modeling of reaction rate acceleration in microdroplets. J. Phys. Chem. A 124, 4984–4989 (2020).
Kozlowski, J. & Zuman, P. Acid–base, hydration–dehydration and keto–enol equilibria in aqueous solutions of α-ketoacids: study by spectroscopy, polarography and linear sweep voltammetry. J. Electroanal. Chem. 343, 43–70 (1992).
Stubbs, R. T., Yadav, M., Krishnamurthy, R. & Springsteen, G. A plausible metal-free ancestral analogue of the Krebs cycle composed entirely of alpha-ketoacids. Nat. Chem. 12, 1016–1022 (2020).
Ma, M. et al. Ultrahigh electrocatalytic conversion of methane at room temperature. Adv. Sci. 4, 1700379 (2017).
Yazdanpour, N. & Sharifnia, S. Photocatalytic conversion of greenhouse gases (CO2 and CH4) using copper phthalocyanine modified TiO2. Sol. Energy Mater. Sol. Cells 118, 1–8 (2013).
Wang, Y. et al. Insight into the synthesis of alcohols and acids in plasma-driven conversion of CO2 and CH4 over copper-based catalysts. Appl. Catal. B 315, 121583–121595 (2022).
Juhl, M. & Lee, J. W. Umpolung reactivity of aldehydes toward carbon dioxide. Angew. Chem. Int. Ed. 57, 12318–12322 (2018).
Miyazaki, M., Shibue, M., Ogino, K., Nakamura, H. & Maeda, H. Enzymatic synthesis of pyruvic acid from acetaldehyde and carbon dioxide. Chem. Commun. 2001, 1800–1801 (2001).
Jestila, J. S. & Uggerud, E. Unimolecular dissociation of anions derived from succinic acid (H2Su) in the gas phase: HSu− and ClMgSu−. Relationship to CO2 fixation. Eur. J. Mass Spectrom. 24, 33–42 (2018).
Schwarz, H. A. & Dodson, R. W. Equilibrium between hydroxyl radicals and thallium(II) and the oxidation potential of OH(aq). J. Phys. Chem. 88, 3643–3647 (1984).
He, J. et al. Probing autoxidation of oleic acid at air–water interface: a neglected and significant pathway for secondary organic aerosols formation. Environ. Res. 212, 113232 (2022).
Xiong, H., Lee, J. K., Zare, R. N. & Min, W. Strong electric field observed at the interface of aqueous microdroplets. J. Phys. Chem. Lett. 11, 7423–7428 (2020).
Zhu, C., Zeng, X. C., Francisco, J. S. & Gladich, I. Hydration, solvation and isomerization of methylglyoxal at the air/water interface: new mechanistic pathways. J. Am. Chem. Soc. 142, 5574–5582 (2020).
Lee, J. K. et al. Spontaneous generation of hydrogen peroxide from aqueous microdroplets. Proc. Natl Acad. Sci. USA 116, 19294–19298 (2019).
Miller, S. L. A production of amino acids under possible primitive Earth conditions. Science 117, 528–529 (1953).
Liu, L., Wei, Q., Zhou, Y. & Ren, X. Using dialkyl amide via forming hydrophobic deep eutectic solvents to separate citric acid from fermentation broth. Green Chem. 22, 2526–2533 (2020).
Chen, Y. X., Wei, Q. F. & Ren, X. L. The effect of hydrophilic amines on hydrothermal liquefaction of macroalgae residue. Bioresour. Technol. 243, 409–416 (2017).
Zhang, X. et al. Rapid monitoring approach for microplastics using portable pyrolysis-mass spectrometry. Anal. Chem. 92, 4656–4662 (2020).
Xing, D. et al. Capture of hydroxyl radicals by hydronium cations in water microdroplets. Angew. Chem. Int. Ed. Engl. 61, e202207587 (2022).
Acknowledgements
We acknowledge financially support from National Natural Science Foundation of China grant no. 21904029 to H.Z. and grant no. 22074026 to J.J. We acknowledge assistance from R. N. Zare in providing key suggestions on manuscript organization and acknowledge C. Gao for discussing the reductive carboxylation mechanism.
Author information
Authors and Affiliations
Contributions
H.Z. and J.J. developed the original ideas presented in the manuscript and supervised the research. H.Z., J.J. and Y. Ju designed the experiments and Y. Ju completed the experiments with assistance from W.W., J.L., G.K., K.Y. and X.W. The reductive carboxylation mechanism was developed by J.J., H.Z., Y. Jiang and J.L. and the data analyses were mainly performed by H.Z., Y. Ju and Y. Jiang. The HPLC analysis was completed by Y. Ju and Y. Jiang. The first draft of the paper was written by Y. Ju, H.Z. and J.J. All authors contributed to finalizing the manuscript. All authors have jointly revised the manuscript and agreed to the published version of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Hong Gil Nam and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Comparison of succinate 7 in CO2- and 13CO2-saturated aqueous microdroplets.
a, Experimental setup; the use of the tube between the sprayer and the MS inlet aimed to avoid the interference of atmosphere. b, Potential intermediates and products from nebulization of 7 in CO2- and 13CO2-saturated water solution.
Extended Data Fig. 2 Comparison of propionic acid in CO2- and 13CO2-saturated aqueous microdroplets.
a, Mass spectra of potential intermediates and products in CO2- and 13CO2-saturated aqueous microdroplets. b, Tandem mass spectra of m/z 145 in the case of CO2-saturated solution. c, Tandem mass spectra of m/z 147 in the case of 13CO2-saturated solution. Nebulization of propionic acid in 13CO2-saturated water solution clearly showed peaks of m/z 102 and m/z 147, which was 1 Da and 2 Da shift compared to those with CO2. This was also consisted with that from nebulization of 7 in CO2- and 13CO2-saturated water solution (Extended Data Fig. 1). The tandem mass spectrum of m/z 147 showed a similar pattern compared to that of m/z 145, which was consistent with the standard 9 (Fig. 3a). This suggested that nebulization of propionic acid could also generate 9 and further supported the assumption that initial decarboxylation of 7 generated propionic acid.
Extended Data Fig. 3 Reductive carboxylation of succinate 7 and α-ketoglutarate 9 in acetonitrile microdroplets.
a, Succinate 7. b, α-ketoglutarate 9. c, Expansion showing spectra for m/z 142-148 and m/z 114-120 from nebulization of 7 and 9 using acetonitrile. d, Expansion showing spectra for m/z 142-148 and m/z 114-120 from nebulization of 7 and 9 using water. When acetonitrile was used as solvent, no corresponding products of m/z 145 and 191, originated from 7 and 9, were observed. This suggested that the reductive carboxylation reactions in aprotic solvent was unfavourable. This supported that the important role of water during the reductive carboxylation. When either water or acetonitrile were used as solvents, the reagent radical anions (7•-, m/z 118; 9•-, m/z 146) were not observed and at least that were lower than the detection limitation. Peaks of m/z 118 and 146 in c and d corresponded to the isotope peaks of reagents because the simulation abundance of these peaks were about 5.4 %. Combing with these results and OH• identification63 suggested that the electrons may be mainly derived from OH− generated by water splitting. In addition, the formation of OH• at the microdroplet surface was also confirmed by other groups66,71.
Extended Data Fig. 4 Effect of high voltage on reductive carboxylation of α-ketoglutarate 9.
a, Reaction with high voltage of −4.5 kV. b, Reaction without high voltage. c, Reaction with the stainless-steel tube grounded. d, Reaction with peek tube instead of the stainless-steel tube. No voltage was used in c and d. The concentration of 9 was 0.1 mM. The product abundances showed no substantial difference.
Extended Data Fig. 5 Effect of high voltage on reductive carboxylation of succinate 7.
a, Reaction with high voltage of −4.5 kV. b, Reaction without high voltage. c, Reaction with the stainless-steel tube grounded. d, Reaction with peek tube instead of the stainless-steel tube. No voltage was used in c and d. The concentration of 7 was 0.1 mM. From Extended Data Fig. 4 and Fig. 5, the abundances for the corresponding products (m/z 191 and m/z 145) showed no substantial difference. This suggested that in absence of high voltage, the products could be also generated when the reagent solution was nebulized.
Supplementary information
Supplementary Information
Supplementary Figs. 1–25, Discussion and Tables 1–5.
Supplementary Data 1
Statistical source data for Table 1.
Supplementary Data 2
Statistical source data for Supplementary Figs. 10, 21 and 25 and Tables 2 and 5.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ju, Y., Zhang, H., Jiang, Y. et al. Aqueous microdroplets promote C–C bond formation and sequences in the reverse tricarboxylic acid cycle. Nat Ecol Evol 7, 1892–1902 (2023). https://doi.org/10.1038/s41559-023-02193-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-023-02193-8