Abstract
Biodiversity time series reveal global losses and accelerated redistributions of species, but no net loss in local species richness. To better understand how these patterns are linked, we quantify how individual species trajectories scale up to diversity changes using data from 68 vegetation resurvey studies of seminatural forests in Europe. Herb-layer species with small geographic ranges are being replaced by more widely distributed species, and our results suggest that this is due less to species abundances than to species nitrogen niches. Nitrogen deposition accelerates the extinctions of small-ranged, nitrogen-efficient plants and colonization by broadly distributed, nitrogen-demanding plants (including non-natives). Despite no net change in species richness at the spatial scale of a study site, the losses of small-ranged species reduce biome-scale (gamma) diversity. These results provide one mechanism to explain the directional replacement of small-ranged species within sites and thus explain patterns of biodiversity change across spatial scales.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The community change and environmental site-level data are available on figshare at https://figshare.com/s/45d71eb77c23c11bc857. The species composition data are available from forestreplot.ugent.be, but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. These data are, however, available from the authors upon request and with the permission of the forestREplot consortium.
Code availability
The R code for all analyses is available on figshare at https://doi.org/10.6084/m9.figshare.10110713.v1.
References
Barnosky, A. D. et al. Has the earth’s sixth mass extinction already arrived? Nature 471, 51–57 (2011).
Díaz, S. et al. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (Advance Unedited Version) (IPBES Secretariat, 2019).
Pereira, H. M., Navarro, L. M. & Martins, I. S. Global biodiversity change: the bad, the good, and the unknown. Annu. Rev. Environ. Resour. 37, 25–50 (2012).
Vellend, M. et al. Global meta-analysis reveals no net change in local-scale plant biodiversity over time. Proc. Natl Acad. Sci. USA 110, 19456–19459 (2013).
Dornelas, M. et al. Assemblage time series reveal biodiversity change but not systematic loss. Science 344, 296–299 (2014).
Vellend, M. et al. Estimates of local biodiversity change over time stand up to scrutiny. Ecology 98, 583–590 (2017).
Newbold, T. et al. Global effects of land use on local terrestrial biodiversity. Nature 520, 45–50 (2015).
Damgaard, C. A critique of the space-for-time substitution practice in community ecology. Trends Ecol. Evol. 34, 416–421 (2019).
Cardinale, B. J., Gonzalez, A., Allington, G. R. H. & Loreau, M. Is local biodiversity declining or not? A summary of the debate over analysis of species richness time trends. Biol. Conserv. 219, 175–183 (2018).
Gonzalez, A. et al. Estimating local biodiversity change: a critique of papers claiming no net loss of local diversity. Ecology 97, 1949–1960 (2016).
Magurran, A. E., Dornelas, M., Moyes, F., Gotelli, N. J. & McGill, B. Rapid biotic homogenization of marine fish assemblages. Nat. Commun. 6, 8405 (2015).
Brown, J. H. On the relationship between abundance and distribution of species. Am. Nat. 124, 255–279 (1984).
Gaston, K. J. The multiple forms of the interspecific abundance–distribution relationship. Oikos 76, 211–220 (1996).
Gaston, K. J. et al. Abundance–occupancy relationships. J. Appl. Ecol. 37, 39–59 (2000).
Schoener, T. W. & Spiller, D. A. High population persistence in a system with high turnover. Nature 330, 474–477 (1987).
Kambach, S. et al. Of niches and distributions: range size increases with niche breadth both globally and regionally but regional estimates poorly relate to global estimates. Ecography (Cop.) 42, 467–477 (2019).
Berendse, F. & Aerts, R. Nitrogen-use-efficiency: a biologically meaningful definition? Funct. Ecol. 1, 293–296 (1987).
Galloway, J. N. et al. Nitrogen cycles: past, present, and future. Biogeochemistry 70, 153–226 (2004).
Aber, J. D. et al. Is nitrogen deposition altering the nitrogen status of northeastern forests? BioScience 53, 375–389 (2003).
Gilliam, F. S. Response of the herbaceous layer of forest ecosystems to excess nitrogen deposition. J. Ecol. 94, 1176–1191 (2006).
Aber, J. et al. Nitrogen saturation in temperate forest ecosystems: hypotheses revisited. BioScience 48, 921–934 (1998).
Tian, D., Wang, H., Sun, J. & Niu, S. Global evidence on nitrogen saturation of terrestrial ecosystem net primary productivity. Environ. Res. Lett. 11, 24012 (2016).
Clark, C. M. & Tilman, D. Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature 451, 712–715 (2008).
Stevens, C. J., Dise, N. B., Mountford, J. O. & Gowing, D. J. Impact of nitrogen deposition on the species richness of grasslands. Science 303, 1876–1879 (2004).
Smith, M. D., Knapp, A. K. & Collins, S. L. A framework for assessing ecosystem dynamics in response to chronic resource alterations induced by global change. Ecology 90, 3279–3289 (2009).
Bobbink, R. et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol. Appl. 20, 30–59 (2010).
Clark, C. M. et al. Potential vulnerability of 348 herbaceous species to atmospheric deposition of nitrogen and sulfur in the United States. Nat. Plants 5, 697–705 (2019).
Ortmann-Ajkai, A. et al. Twenty-years’ changes of wetland vegetation: effects of floodplain-level threats. Wetlands 38, 591–604 (2018).
Hernández, D. L. et al. Nitrogen pollution is linked to US listed species declines. BioScience 66, 213–222 (2016).
Simkin, S. M. et al. Conditional vulnerability of plant diversity to atmospheric nitrogen deposition across the United States. Proc. Natl Acad. Sci. USA 113, 4086–4091 (2016).
Sonkoly, J. et al. Do large-seeded herbs have a small range size? The seed mass–distribution range trade-off hypothesis. Ecol. Evol. 7, 11204–11212 (2017).
Bartelheimer, M. & Poschlod, P. Functional characterizations of Ellenberg indicator values—a review on ecophysiological determinants. Funct. Ecol. 30, 506–516 (2016).
Grime, J. P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 111, 1169–1194 (1977).
Grotkopp, E., Rejmánek, M. & Rost, T. L. Toward a causal explanation of plant invasiveness: seedling growth and life-history strategies of 29 pine (Pinus) species. Am. Nat. 159, 396–419 (2002).
Fenner, M. & Thompson, K. The Ecology of Seeds (Cambridge Univ. Press, 2005).
Van der Veken, S., Bellemare, J., Verheyen, K. & Hermy, M. Life-history traits are correlated with geographical distribution patterns of western European forest herb species. J. Biogeogr. 34, 1723–1735 (2007).
McKinney, M. L. & Lockwood, J. L. Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 14, 450–453 (1999).
Hanski, I. Dynamics of regional distribution: the core and satellite species hypothesis. Oikos 38, 210–221 (1982).
Wright, D. H. Correlations between incidence and abundance are expected by chance. J. Biogeogr. 18, 463–466 (1991).
Mason, H. L. The edaphic factor in narrow endemism. I. The nature of environmental influences. Madroño 8, 209–226 (1946).
Sandel, B. S. et al. The influence of Late Quaternary climate-change velocity on species endemism. Science 334, 660–664 (2011).
Hubbell, S. P. The Unified Neutral Theory of Biodiversity and Biogeography (MPB-32) (Princeton Univ. Press, 2001).
Suding, K. N. et al. Functional- and abundance-based mechanisms explain diversity loss due to N fertilization. Proc. Natl Acad. Sci. USA 102, 4387–4392 (2005).
Rabinowitz, D., Cairns, S. & Dillon, T. in Conservation Biology: The Science of Scarcity and Diversity (ed. Soulé, M. E.) 182–204 (Sinauer Associates, 1986).
Köckemann, B., Buschmann, H. & Leuschner, C. The relationships between abundance, range size and niche breadth in Central European tree species. J. Biogeogr. 36, 854–864 (2009).
Thompson, K., Hodgson, J. G. & Gaston, K. J. Abundance–range size relationships in the herbaceous flora of central England. J. Ecol. 86, 439–448 (1998).
Verheyen, K. et al. Driving factors behind the eutrophication signal in understorey plant communities of deciduous temperate forests. J. Ecol. 100, 352–365 (2012).
Dirnböck, T. et al. Forest floor vegetation response to nitrogen deposition in Europe. Glob. Change Biol. 20, 429–440 (2014).
Bernhardt-Römermann, M. et al. Drivers of temporal changes in temperate forest plant diversity vary across spatial scales. Glob. Change Biol. 21, 3726–3737 (2015).
Borer, E. T. et al. Herbivores and nutrients control grassland plant diversity via light limitation. Nature 508, 517–520 (2014).
Hautier, Y., Niklaus, P. A. & Hector, A. Competition for light causes plant biodiversity loss after eutrophication. Science 324, 636–638 (2009).
De Frenne, P. et al. Global buffering of temperatures under forest canopies. Nat. Ecol. Evol. 3, 744–749 (2019).
De Frenne, P. et al. Microclimate moderates plant responses to macroclimate warming. Proc. Natl Acad. Sci. USA 110, 18561–18565 (2013).
Amann, M. et al. Progress towards the Achievement of the EU’s Air Quality and Emissions Objectives (IIASA, 2018).
Storkey, J. et al. Grassland biodiversity bounces back from long-term nitrogen addition. Nature 528, 401–404 (2015).
Isbell, F., Tilman, D., Polasky, S., Binder, S. & Hawthorne, P. Low biodiversity state persists two decades after cessation of nutrient enrichment. Ecol. Lett. 16, 454–460 (2013).
Verheyen, K. et al. Combining biodiversity resurveys across regions to advance global change research. BioScience 67, 73–83 (2016).
Peterken, G. F. Natural Woodland: Ecology and Conservation in Northern Temperate Regions (Cambridge Univ. Press, 1996).
Beck, J., Takano, H., Ballesteros-Mejia, L., Kitching, I. J. & McCain, C. M. Field sampling is biased against small-ranged species of high conservation value: a case study on the sphingid moths of East Africa. Biodivers. Conserv. 27, 3533–3544 (2018).
Verheyen, K. et al. Observer and relocation errors matter in resurveys of historical vegetation plots. J. Veg. Sci. 29, 812–823 (2018).
Kopecký, M. & Macek, M. Vegetation resurvey is robust to plot location uncertainty. Divers. Distrib. 21, 322–330 (2015).
GBIF Occurrence Download (GBIF, accessed 18 January 2019); https://doi.org/10.15468/dl.l1r0yg
Chamberlain, S. scrubr: clean biological occurrence records (R package v.0.1, 2016).
Gaston, K. J. & Fuller, R. A. The sizes of species’ geographic ranges. J. Appl. Ecol. 46, 1–9 (2009).
Isaac, N. J. B. & Pocock, M. J. O. Bias and information in biological records. Biol. J. Linn. Soc. 115, 522–531 (2015).
Meyer, C., Weigelt, P. & Kreft, H. Multidimensional biases, gaps and uncertainties in global plant occurrence information. Ecol. Lett. 19, 992–1006 (2016).
Hultén, E., et al. Atlas of North European Vascular Plants North of the Tropic of Cancer (Koeltz Scientific, 1986).
Meusel, H., Jäger, E. J. & Weinert, E. Vergleichende Chorologie der Zentraleuropaischen Flora (Gustav Fischer, 1965).
Berg, C., Welk, E. & Jäger, E. J. Revising Ellenberg’s indicator values for continentality based on global vascular plant species distribution. Appl. Veg. Sci. 20, 482–493 (2017).
Stevens, C. J. et al. Ecosystem responses to reduced and oxidised nitrogen inputs in European terrestrial habitats. Environ. Pollut. 159, 665–676 (2011).
van den Berg, L. J. L. et al. Evidence for differential effects of reduced and oxidised nitrogen deposition on vegetation independent of nitrogen load. Environ. Pollut. 208, 890–897 (2016).
Dorland, E. et al. Differential effects of oxidised and reduced nitrogen on vegetation and soil chemistry of species-rich acidic grasslands. Water, Air, Soil Pollut. 224, 1664 (2013).
Gauss, M. et al. EMEP MSC-W Model Performance for Acidifying and Eutrophying Components, Photo-oxidants and Particulate Matter in 2017 (Supplementary Material to EMEP Status Report, 2019).
Asman, W. A. H. Factors influencing local dry deposition of gases with special reference to ammonia. Atmos. Environ. 32, 415–421 (1998).
Ellenberg, H., Weber, H. E., Düll, R., Wirth, V. & Werner, W. Zeigerwerte von Pflanzen in Mitteleuropa (Goltze, 2001).
Diekmann, M. Species indicator values as an important tool in applied plant ecology—a review. Basic Appl. Ecol. 4, 493–506 (2003).
McElreath, R. Statistical Rethinking: A Bayesian Course with Examples in R and Stan (Chapman and Hall, CRC, 2018).
Peterson, R. A. bestNormalize: normalizing transformation functions (R package v.1.2.0, 2018).
Olson, D. M. et al. Terrestrial ecoregions of the world: a new map of life on Earth: a new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. BioScience 51, 933–938 (2001).
Hansen, M. C. et al. High-resolution global maps of 21st-century forest cover change. Science 342, 850–853 (2013).
Pearl, J. Causality 2nd edn (Cambridge Univ. Press, 2009).
Acknowledgements
This paper is an outcome of the sREplot working group supported by sDiv, the Synthesis Centre of the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig (DFG FZT 118). P.D.F. and P.V. received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (ERC Starting Grant FORMICA 757833). K.V. received funding through ERC Consolidator Grant PASTFORWARD 614839. M.K. and M. Macek were supported by the Czech Academy of Sciences (grant no. RVO 67985939). F.M. was supported by the Slovak Research and Development Agency (grant no. APVV-15-0270). R.H, M.C. and O.V. were supported by the grant agency of the Czech Republic (grant no. 17-09283S) and Czech Academy of Sciences (grant no. RVO 67985939). T.N. was supported by the Slovenian Research Agency (grant no. J4-1765). I.B. was supported by grant no. EFOP-3.6.1-16-2016-00018. R.P. was supported by a grant from the National Science Centre, Poland (no. 2016/20/S/NZ800428). B.T. was financed by the Higher Education Institutional Excellence Program of the Ministry for Innovation and Technology in Hungary, within the framework of the third thematic programme of the University of Pécs.
Author information
Authors and Affiliations
Contributions
I.R.S., D.M.W. and L.B. conceived the study, with input from the sREplot working group (M.B.-R., A.D.B., J.B., P.D.F., R.H., U.J., J.L., F.M., K.V. and M.W.). I.R.S. performed the analyses, with input from D.M.W. and L.B. I.R.S., D.M.W. and L.B. wrote the manuscript, with input and contributions from M.B.-R., A.D.B., J.B., P.D.F., R.H., U.J., J.L., F.M., K.V., M.W., H.M.P., P.V., A.O.-A., R.P., I.B., M.C., G.D., T. Dirnböck, T. Durak, W.S., T.H., F.H.S., B.J., M.K., M. Macek, M. Malicki, T.N., T.A.N., P.P., K.R., T.S., K.Ś., B.T., H.V.C. and O.V. The authorship order was determined as follows: (1) core authors, (2) sREplot participants (alphabetical) and other major contributors and (3) authors contributing community composition data and to an advanced version of the manuscript (alphabetical).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
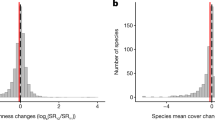
Extended Data Fig. 1 Change in species numbers.
Frequency distribution of the difference in species numbers between the resurvey and baseline survey.
Extended Data Fig. 2 Change in nitrophilous species.
a, Frequency distribution of Ellenberg indicator values for nitrogen (eivN) across species. b, Frequency distribution of the mean eivN of extinct (dark green) and colonizing (light green) species.
Extended Data Fig. 3 Range sizes.
a, Frequency distribution of range sizes measured as area of occupancy (AOO) from GBIF point occurrence records. b, Spearman correlation plot of AOO and extent of occurrence (EOO) range sizes from digitized range maps. Points colored in magenta identify continental species. Correlation coefficient with and without continental species is: ρ = .71 and ρ = .74, respectively.
Extended Data Fig. 4 Correlations between predictor variables.
the year of the baseline survey (t1), time between surveys (∆t), cumulative N deposition between 1900 and the year of the baseline survey (Nt1) and intercensus cumulative nitrogen deposition (∆N).
Extended Data Fig. 5 Directed acyclic graph.
Directed acyclic graph of hypothesized causal links between predictor and response variables. Cumulative N deposition at the year of the baseline (Nt1), intercensus cumulative N-deposition (∆N) and time between surveys (∆t) directly influence the outcome (extinction probability, E). Year of the baseline survey (t1) directly influences ∆t and Nt1: the earlier the baseline survey, the longer the time between surveys; the earlier the baseline survey, the lower the cumulative N deposition at the year of the baseline survey. To estimate the direct effect of ∆N, it is sufficient to include ∆t as a covariate. This closes the backdoor81 through t1 (t1 →Nt1 → E) and as a result differences in baseline year do not confound the effect of ∆N.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3 and Tables 1–7.
Rights and permissions
About this article
Cite this article
Staude, I.R., Waller, D.M., Bernhardt-Römermann, M. et al. Replacements of small- by large-ranged species scale up to diversity loss in Europe’s temperate forest biome. Nat Ecol Evol 4, 802–808 (2020). https://doi.org/10.1038/s41559-020-1176-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-020-1176-8
This article is cited by
-
Anthropogenic climate and land-use change drive short- and long-term biodiversity shifts across taxa
Nature Ecology & Evolution (2024)
-
Distinct hybridization modes in wide- and narrow-ranged lineages of Causonis (Vitaceae)
BMC Biology (2023)
-
Power laws in species’ biotic interaction networks can be inferred from co-occurrence data
Nature Ecology & Evolution (2023)
-
Regional occupancy increases for widespread species but decreases for narrowly distributed species in metacommunity time series
Nature Communications (2023)
-
Nitrogen deposition causes eutrophication in bryophyte communities in central and northern European forests
Annals of Forest Science (2022)