Abstract
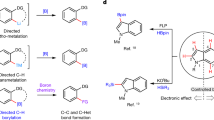
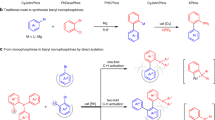
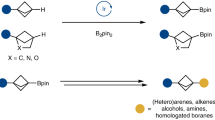
Given the important role played by 2-hydroxybiaryls in organic, medicinal and materials chemistry, concise methods for the synthesis of this common motif are extremely valuable. In seeking to extend the lexicon of synthetic chemists in this regard, we have developed an expedient and general strategy for the ortho-arylation of phenols and naphthols using readily available boronic acids. Our methodology relies on in situ generation of a uniquely reactive Bi(v) arylating agent from a bench-stable Bi(iii) precursor via telescoped B–to–Bi transmetallation and oxidation. By exploiting reactivity that is orthogonal to conventional metal-catalysed manifolds, diverse aryl and heteroaryl partners can be rapidly coupled to phenols and naphthols under mild conditions. Following arylation, high-yielding recovery of the Bi(iii) precursor allows for its efficient re-use in subsequent reactions. Mechanistic interrogation of each key step of the methodology informs its practical application and provides fundamental insight into the underexploited reactivity of organobismuth compounds.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers 1904059 (1-OTs), 1904060 (2a), 1904061 (2b), 1904062 (2c), 1904063 (2e), 1904064 (2g), 1904065 (2h), 1904066 (2i), 1904069 (2j), 1904067 (2k), 1904068 (2m), 1904070 (2u), 1904071 (2v), 1904072 (9) and 1904073 (50). Copies of the data can be obtained free of charge at www.ccdc.cam.ac.uk/data_request/cif. The authors declare that all other data supporting the findings of this study are available within the paper and its Supplementary information.
References
Ibrahim, S. R. M. & Mohamed, G. A. Naphthylisoquinoline alkaloids potential drug leads. Fitoterapia 106, 194–225 (2015).
Teponno, R. B., Kusari, S. & Spiteller, M. Recent advances in research on lignans and neolignans. Nat. Prod. Rep. 33, 1044–1092 (2016).
Quideau, S., Deffieux, D., Douat-Casassus, C. & Pouysegu, L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 50, 586–621 (2011).
Stockert, A. L. & Hill, M. in Bioactive Components, Diet and Medical Treatment in Cancer Prevention (eds Waly, M. & Rahman, M.) Ch. 2, 25–50 (Springer, 2018).
Parmar, D., Sugiono, E., Raja, S. & Rueping, M. Complete field guide to asymmetric BINOL-phosphate derived Brønsted acid and metal catalysis: history and classification by mode of activation; Brønsted acidity, hydrogen bonding, ion pairing, and metal phosphates. Chem. Rev. 114, 9047–9153 (2014).
Reid, J. P. & Goodman, J. M. Selecting chiral BINOL‐derived phosphoric acid catalysts: general model to identify steric features essential for enantioselectivity. Chem. Eur. J. 23, 14248–14260 (2017).
Cramer, J., Sager, C. P. & Ernst, B. Hydroxyl groups in synthetic and natural-product-derived therapeutics: a perspective on a common functional group. J. Med. Chem. 62, 8915–8930 (2019).
Zhang, H. et al. Molecular determinants of Magnolol targeting both RXRα and PPARγ. PLOS ONE 6, e28253 (2011).
Marchais-Oberwinkler, S. et al. New drug-like hydroxyphenylnaphthol steroidomimetics as potent and selective 17β-hydroxysteroid dehydrogenase type 1 inhibitors for the treatment of estrogen-dependent diseases. J. Med. Chem. 54, 534–547 (2010).
Gross, K. C. & Seybold, P. G. Substituent effects on the physical properties and pK a of phenol. Int. J. Quantum Chem. 85, 569–579 (2001).
Bosmans, V. et al. Probing through-space polar–π interactions in 2,6-diarylphenols. J. Org. Chem. 84, 3632–3637 (2019).
Hassan, J., Sévignon, M., Gozzi, C., Schulz, E. & Lemaire, M. Aryl–aryl bond formation one century after the discovery of the Ullmann reaction. Chem. Rev. 102, 1359–1470 (2002).
Huang, Z. & Lumb, J.-P. Phenol-directed C–H functionalization. ACS Catal. 9, 521–555 (2019).
Alberico, D., Scott, M. E. & Lautens, M. Aryl–aryl bond formation by transition-metal-catalyzed direct arylation. Chem. Rev. 107, 174–238 (2007).
Zhao, X., Yeung, C. S. & Dong, V. M. Palladium-catalyzed ortho-arylation of O-phenylcarbamates with simple arenes and sodium persulfate. J. Am. Chem. Soc. 132, 5837–5844 (2010).
Bedford, R. B., Webster, R. L. & Mitchell, C. J. Palladium-catalysed ortho-arylation of carbamate-protected phenols. Org. Biomol. Chem. 7, 4853–4857 (2009).
Bedford, R. B. et al. Palladium-catalyzed ortho-arylation of carbamate-protected estrogens. J. Org. Chem. 81, 3473–3478 (2016).
Xiao, B. et al. Pd(ii)-catalyzed C−H activation/aryl–aryl coupling of phenol esters. J. Am. Chem. Soc. 132, 468–469 (2010).
Ackermann, L., Diers, E. & Manvar, A. Ruthenium-catalyzed C–H bond arylations of arenes bearing removable directing groups via six-membered ruthenacycles. Org. Lett. 14, 1154–1157 (2012).
Gu, S., Chen, C. & Chen, W. Ortho-functionalization of 2-phenoxypyrimidines via palladium-catalyzed C–H bond activation. J. Org. Chem. 74, 7203–7206 (2009).
Bedford, R. B. et al. Simple rhodium–chlorophosphine pre-catalysts for the ortho-arylation of phenols. Chem. Commun. 990–992 (2008).
Bajracharya, G. B. & Daugulis, O. Direct transition-metal-free intramolecular arylation of phenols. Org. Lett. 10, 4625–4628 (2008).
Truong, T. & Daugulis, O. Divergent reaction pathways for phenol arylation by arynes: synthesis of helicenes and 2-arylphenols. Chem. Sci. 4, 531–535 (2013).
Bedford, R. B. & Limmert, M. E. Catalytic intermolecular ortho-arylation of phenols. J. Org. Chem. 68, 8669–8682 (2003).
Oi, S., Watanabe, S., Fukita, S. & Inoue, Y. Rhodium-HMPT-catalyzed direct ortho-arylation of phenols with aryl bromides. Tetrahedron Lett. 44, 8665–8668 (2003).
Bedford, R. B., Haddow, M. F., Webster, R. L. & Mitchell, C. J. The catalytic ortho-arylation of tyrosine. Org. Biomol. Chem. 7, 3119–3127 (2009).
Barton, D. H. R., Lester, D. J., Motherwell, W. B. & Papoula, M. T. B. Observations on the cleavage of the bismuth–carbon bond in BiV compounds: a new arylation reaction. J. Chem. Soc. Chem. Commun. 246–247 (1980).
Barton, D. H. R. et al. Comparative arylation reactions with pentaphenylbismuth and with triphenylbismuth carbonate. J. Chem. Soc. Chem. Commun. 827–829 (1980).
Gagnon, A., Dansereau, J. & Le Roch, A. Organobismuth reagents: synthesis, properties and applications in organic synthesis. Synthesis 49, 1707–1745 (2017).
Gagnon, A, Benoit, E. & Le Roch, A. Sci. Synth., Knowl. Updates 4, 2–112 (2018).
Suzuki, H. et al. Organobismuth Chemistry 1st edn (Elsevier, 2001).
Balsane, K. E., Gund, S. H. & Nagarkar, J. M. Atom economic palladium catalyzed novel approach for arylation of benzothiazole and benzoxazole with triarylbismuth reagents via C-H activation. Catal. Commun. 89, 29–33 (2017).
Fedorov, A. Y. & Finet, J.-P. Synthesis and reactivity of pentavalent biphenyl-2,2′-ylenebismuth derivatives. J. Chem. Soc., Perkin Trans. 1, 3775–3778 (2000).
Suzuki, H., Murafuji, T. & Azuma, N. Synthesis and reactions of some new heterocyclic bismuth-(iii) and -(v) compounds. 5,10-Dihydrodibenzo[b,e]bismine and related systems. J. Chem. Soc. Perkin Trans. 1, 1593–1600 (1992).
Sakurai, N. & Mukaiyama, T. A new preparative method of aryl sulfonate esters by using cyclic organobismuth reagents. Heterocycles 74, 771–790 (2007).
Murafuji, T. et al. Bismuth heterocycles based on a diphenyl sulfone scaffold: synthesis and substituent effect on the antifungal activity against Saccharomyces cerevisiae. Eur. J. Med. Chem. 46, 519–525 (2011).
Murafuji, T., Nagasue, M., Tashiro, Y., Sugihara, Y. & Azuma, N. Structural characteristics of aryloxybismuthanes stabilized by hypervalent bond formation. Synthesis, incorporation of 4-methoxyphenol through hydrogen bonding, and crystal supramolecularity. Organometallics 19, 1003–1007 (2000).
Ohkata, K., Takemoto, S., Ohnishi, M. & Akiba, K. Synthesis and chemical behaviors of 12-substituted dibenz[c,f][1,5]azastibocine and dibenz[c,f][1,5]azabismocine derivatives: evidences of 10-Pn-4 type hypervalent interaction. Tetrahedron Lett. 30, 4841–4844 (1989).
Ikegami, T. & Suzuki, H. A stabilized triarylbismuthane imide: synthesis and first X-ray structure analysis. Organometallics 17, 1013–1017 (1998).
Matano, Y., Begum, S. A., Miyamatsu, T. & Suzuki, H. A new and efficient method for the preparation of bismuthonium and telluronium salts using aryl- and alkenylboronic acids. First observation of the chirality at bismuth in an asymmetrical bismuthonium salt. Organometallics 17, 4332–4334 (1998).
Yoshihiro, M., Takashi, M. & Hitomi, S. Synthesis and reaction of unsymmetrical tetraarylbismuthonium salts. First isolation of bismuthonium salts bearing all different aryl groups. Chem. Lett. 27, 127–128 (1998).
Matano, Y., Begum, S. A., Miyamatsu, T. & Suzuki, H. Synthesis and stereochemical behavior of unsymmetrical tetraarylbismuthonium salts. Organometallics 18, 5668–5681 (1999).
Matano, Y., Begum, S. A. & Suzuki, H. A new synthesis of triarylbismuthanes via directed ligand coupling of oxazoline-substituted tetraarylbismuthonium salts: synthesis of polystyrenes bearing the diarylbismuthino group. Synthesis 1081–1085 (2001).
Matano, Y. & Imahori, H. A new, efficient method for direct α-alkenylation of β-dicarbonyl compounds and phenols using alkenyltriarylbismuthonium salts. J. Org. Chem. 69, 5505–5508 (2004).
Stavila, V., Thurston, J. H., Prieto-Centurión, D. & Whitmire, K. H. A new methodology for synthesis of aryl bismuth compounds: arylation of bismuth(iii) carboxylates by sodium tetraarylborate salts. Organometallics 26, 6864–6866 (2007).
Dostál, L. et al. From stiba- and bismaheteroboroxines to N,C,N-chelated diorganoantimony(iii) and bismuth(iii) cations—an unexpected case of aryl group migration. Inorg. Chem. 54, 6010–6019 (2015).
Cox, P. A., Leach, A. G., Campbell, A. D. & Lloyd-Jones, G. C. Protodeboronation of heteroaromatic, vinyl, and cyclopropyl boronic acids: pH–rate profiles, autocatalysis, and disproportionation. J. Am. Chem. Soc. 138, 9145–9157 (2016).
Cox, P. A. et al. Base-catalyzed aryl-B(OH)2 protodeboronation revisited: from concerted proton transfer to liberation of a transient aryl anion. J. Am. Chem. Soc. 139, 13156–13165 (2017).
Kozminskaya, T. K., Nadj, M. M. & Kocheshkov, K. A. The synthesis of organo-bismuth compounds of the type R3Bi by the method of double diazonium salts. Zh. Obshch. Khim. 16, 891–896 (1946).
Matano, Y. et al. Water-soluble non-ionic triarylbismuthanes. First synthesis and properties. J. Chem. Soc., Perkin Trans. 1, 2511–2518 (1998).
Hébert, M. et al. Synthesis of highly functionalized triarylbismuthines by functional group manipulation and use in palladium- and copper-catalyzed arylation reactions. J. Org. Chem. 81, 5401–5416 (2016).
Preda, A. M. et al. Heteroaryl bismuthines: a novel synthetic concept and metal⋯π heteroarene interactions. Dalton Trans. 46, 8269–8278 (2017).
Petiot, P. & Gagnon, A. Palladium‐catalyzed cross‐coupling reaction of functionalized aryl‐ and heteroarylbismuthanes with 2‐halo(or 2‐triflyl)azines and ‐diazines. Eur. J. Org. Chem. 24, 5282–5289 (2013).
Merck and Co. O-Heteroaryl, O-alkylheteroaryl, O-alkenylheteroaryl and O-alkynylheteroarylmacrolides having immunosuppressive activity. US patent US5252732 (1993).
Urgin, K. et al. Advanced preparation of functionalized triarylbismuths and triheteroaryl-bismuths: new scope and alternatives. Tetrahedron Lett. 53, 1894–1896 (2012).
Kinzel, T., Zhang, Y. & Buchwald, S. L. A new palladium precatalyst allows for the fast Suzuki−Miyaura coupling reactions of unstable polyfluorophenyl and 2-heteroaryl boronic acids. J. Am. Chem. Soc. 132, 14073–14075 (2010).
Chen, L., Sanchez, D. R., Zhang, B. & Carrow, B. P. “Cationic” Suzuki–Miyaura coupling with acutely base-sensitive boronic acids. J. Am. Chem. Soc. 139, 12418–12421 (2017).
Babudri, F., Farinola, G. M., Naso, F. & Ragni, R. Fluorinated organic materials for electronic and optoelectronic applications: the role of the fluorine atom. Chem. Commun. 1003–1022 (2007).
Fedorov, A., Combes, S. & Finet, J.-P. Influence of the steric hindrance of the aryl group of pentavalent triarylbismuth derivatives in ligand coupling reactions. Tetrahedron 55, 1341–1352 (1999).
Barton, D. H. R. et al. The chemistry of pentavalent organobismuth reagents: Part X. Studies on the phenylation and oxidation of phenols. Tetrahedron 43, 323–332 (1987).
Evano, G., Blanchard, N. & Toumi, M. Copper-mediated coupling reactions and their applications in natural products and designed biomolecules synthesis. Chem. Rev. 108, 3054–3131 (2008).
Qiao, J. X. & Lam, P. Y. S. Copper-promoted carbon-heteroatom bond cross-coupling with boronic acids and derivatives. Synthesis 6, 829–856 (2011).
Crifar, C., Petiot, P., Ahmad, T. & Gagnon, A. Synthesis of highly functionalized diaryl ethers by copper‐mediated O‐arylation of phenols using trivalent arylbismuth reagents. Chem. Eur. J. 20, 2755–2760 (2014).
Barton, D. H. R. et al. Pentavalent organobismuth reagents. Part 2. The phenylation of phenols. J. Chem. Soc. Perkin Trans. 1, 2657–2665 (1985).
Hansch, C., Leo, A. & Taft, R. W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 97, 165–195 (1991).
Takahata, Y. & Chong, D. P. Estimation of Hammett sigma constants of substituted benzenes through accurate density-functional calculation of core-electron binding energy shifts. Int. J. Quantum Chem. 103, 509–515 (2005).
Selassie, C. & Verma, R. P. Burger’s Medicinal Chemistry, Drug Discovery, and Development 6th edn, Vol. 1 (John Wiley & Sons, 2003).
Sofia, M. J. et al. o-Phenylphenols: potent and orally active leukotriene B4 receptor antagonists. J. Med. Chem. 36, 3978–3981 (1993).
Sawyer, J. S. et al. Synthetic and structure/activity studies on acid-substituted 2-arylphenols: discovery of 2-[2-propyl-3-[3-[2-ethyl-4-(4-fluorophenyl)-5-hydroxyphenoxy]-propoxy]phenoxy]benzoic acid, a high-affinity leukotriene B4 receptor antagonist. J. Med. Chem. 38, 4411–4432 (1995).
Worm, K., Zhou, Q. J., Stabley, G. J., DeHaven, R. N. & Dolle, R. E. Biaryl cannabinoid mimetics—synthesis and structure–activity relationship. Bioorg. Med. Chem. Lett. 17, 3652–3656 (2007).
Zhang, L. et al. Highly regio- and chemoselective oxidative C–H/C–H cross-couplings of anilines and phenols enabled by a co-oxidant-free Rh(i)/Zn(NTf2)2/air catalytic system. ACS Catal. 9, 5358–5364 (2019).
Hu, Z. & Liu, G. Rhodium(iii)‐catalyzed cascade redox‐neutral C–H functionalization and aromatization: synthesis of unsymmetrical ortho‐biphenols. Adv. Synth. Catal. 359, 1643–1648 (2017).
Xiao, B. et al. Synthesis of dibenzofurans via palladium-catalyzed phenol-directed C–H activation/C–O cyclization. J. Am. Chem. Soc. 133, 9250–9253 (2011).
Ciana, C.-L., Phipps, R. J., Brandt, J. R., Meyer, F.-M. & Gaunt, M. J. A highly para‐selective copper(ii)‐catalyzed direct arylation of aniline and phenol derivatives. Angew. Chem. Int. Ed. 50, 458–462 (2011).
Ivanova, A. et al. Synthesis, functionalization and biological activity of arylated derivatives of (+)-estrone. Bioorg. Med. Chem. 25, 949–962 (2017).
Mewshaw, R. E. et al. ERβ Ligands. 3. Exploiting two binding orientations of the 2-phenylnaphthalene scaffold to achieve ERβ selectivity. J. Med. Chem. 48, 3953–3979 (2005).
Marchais-Oberwinkler, S. et al. Substituted 6-phenyl-2-naphthols. Potent and selective nonsteroidal inhibitors of 17β-hydroxysteroid dehydrogenase type 1 (17β-HSD1): design, synthesis, biological evaluation, and pharmacokinetics. J. Med. Chem. 51, 4685–4698 (2008).
Yin, S.-F., Maruyama, J., Yamashita, T. & Shimada, S. Efficient fixation of carbon dioxide by hypervalent organobismuth oxide, hydroxide, and alkoxide. Angew. Chem. Int. Ed. 47, 6590–6593 (2008).
Qiu, R. et al. Synthesis and structure of binuclear O/S‐bridged organobismuth complexes and their cooperative catalytic effect on CO2 fixation. ChemPlusChem 77, 404–410 (2012).
Carrow, B. P. & Hartwig, J. F. Distinguishing between pathways for transmetalation in Suzuki–Miyaura reactions. J. Am. Chem. Soc. 133, 2116–2119 (2011).
Lennox, A. J. J. & Lloyd-Jones, G. C. Transmetallation in Suzuk–Miyaura coupling: the fork in the trail. Angew. Chem. Int. Ed. 52, 7362–7370 (2013).
Thomas, A. A. & Denmark, S. E. Pre-transmetalation intermediates in the Suzuki–Miyaura reaction revealed: the missing link. Science 352, 329–332 (2016).
Matano, Y. & Nomura, H. Dimeric triarylbismuthane oxide: a novel efficient oxidant for the conversion of alcohols to carbonyl compounds. J. Am. Chem. Soc. 123, 6443–6444 (2001).
Tetrahedron Organic Chemistry Series (ed. Finet, J.-P.) Vol. 18, Ch. 2 (Elsevier, 1998).
Askari, M. S., Esguerra, K. V. N., Lumb, J.-P. & Ottenwaelder, X. A biomimetic mechanism for the copper-catalyzed aerobic oxygenation of 4-tert-butylphenol. Inorg. Chem. 54, 8665–8672 (2015).
Itoh, S. & Fukuzumi, S. Monooxygenase activity of type 3 copper proteins. Acc. Chem. Res. 40, 592–600 (2007).
Hoppe, S. & Whitmire, K. H. Synthesis and structure of pentavalent bismuth(v) alkoxides and ligand redistribution equilibria in solution. Organometallics 17, 1347–1354 (1998).
Ozanne‐Beaudenon, A. & Quideau, S. Regioselective hypervalent‐iodine(iii)‐mediated dearomatizing phenylation of phenols through direct ligand coupling. Angew. Chem. Int. Ed. 44, 7065–7069 (2005).
Pouységu, L., Deffieux, D. & Quideau, S. Hypervalent iodine-mediated phenol dearomatization in natural product synthesis. Tetrahedron 66, 2235–2261 (2010).
Barton, D. H. R., Donnelly, D. M. X., Guiry, P. J. & Finet, J.-P. ortho-Arylation of 3,5-di-tert-butylphenol with aryllead(iv) derivatives: a facile synthesis of sterically hindered phenols. J. Chem. Soc. Perkin Trans. 1, 2921–2926 (1994).
Mihailović, M. L., Čeković, Z. & Mathes, B. M. in Encyclopedia of Reagents for Organic Synthesis (John Wiley & Sons, 2005); https://doi.org/10.1002/047084289X.rl006.pub2
Taylor, R. Electrophilic Aromatic Substitution (John Wiley & Sons, 1990).
Mirica, L. M. et al. Tyrosinase reactivity in a model complex: an alternative hydroxylation mechanism. Science 308, 1890–1892 (2005).
Fujieda, N. et al. Activation mechanism of melB tyrosinase from Aspergillus oryzae by acidic treatment. J. Biol. Inorg. Chem. 18, 19–26 (2013).
Combes, S. & Finet, J.-P. On the exclusion of radical species in the ligand coupling reactions with pentavalent triarylbismuth derivatives. Tetrahedron 55, 3377–3386 (1999).
Acknowledgements
This work was supported by The University of Nottingham.
Author information
Authors and Affiliations
Contributions
M.J. and L.T.B. conceived this work. M.J. and L.M. performed the experiments and analysed the data. W.L. acquired and solved X-ray diffraction data. L.T.B. wrote the manuscript with input from M.J. and L.M.
Corresponding author
Ethics declarations
Competing interests
Bismacycle 1-OTs has been made commercially available via Key Organics. The sales revenue that is returned to the University of Nottingham covers the costs of commercialization; the authors do not receive profit from any of the sales that are made.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Synthetic procedures, characterization data, crystallographic data tables and NMR spectra.
Crystallographic data
Crystallographic data for compound 1-OTs. CCDC reference 1904059.
Crystallographic data
Crystallographic data for compound 2a. CCDC reference 1904060.
Crystallographic data
Crystallographic data for compound 2b. CCDC reference 1904061.
Crystallographic data
Crystallographic data for compound 2c. CCDC reference 1904062.
Crystallographic data
Crystallographic data for compound 2e. CCDC reference 1904063.
Crystallographic data
Crystallographic data for compound 2g. CCDC reference 1904064.
Crystallographic data
Crystallographic data for compound 2h. CCDC reference 1904065.
Crystallographic data
Crystallographic data for compound 2i. CCDC reference 1904066.
Crystallographic data
Crystallographic data for compound 2j. CCDC reference 1904069.
Crystallographic data
Crystallographic data for compound 2k. CCDC reference 1904067.
Crystallographic data
Crystallographic data for compound 2m. CCDC reference 1904068.
Crystallographic data
Crystallographic data for compound 2u. CCDC reference 1904070.
Crystallographic data
Crystallographic data for compound 2v. CCDC reference 1904071.
Crystallographic data
Crystallographic data for compound 9. CCDC reference 1904072.
Crystallographic data
Crystallographic data for compound 50. CCDC reference 1904073.
Rights and permissions
About this article
Cite this article
Jurrat, M., Maggi, L., Lewis, W. et al. Modular bismacycles for the selective C–H arylation of phenols and naphthols. Nat. Chem. 12, 260–269 (2020). https://doi.org/10.1038/s41557-020-0425-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-0425-4
This article is cited by
-
Electrochemical synthesis of biaryls by reductive extrusion from N,N’-diarylureas
Nature Communications (2023)
-
meta-Selective C–H arylation of phenols via regiodiversion of electrophilic aromatic substitution
Nature Chemistry (2023)
-
Heavy metal orchestration
Nature Chemistry (2020)