Abstract
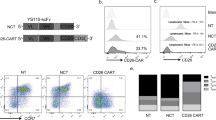
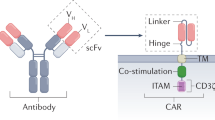
Therapies employing chimeric antigen receptor T cells (CAR-T cells) targeting tumour-associated antigens (TAAs) can lead to on-target–off-tumour toxicity and to resistance, owing to TAA expression in normal tissues and to TAA expression loss in tumour cells. These drawbacks can be circumvented by CAR-T cells targeting tumour-specific driver gene mutations, such as the four-nucleotide duplication in the oncogene nucleophosmin (NPM1c), which creates a neoepitope presented by the human leukocyte antigen with the A2 serotype (HLA-A2) that has been observed in about 35% of patients with acute myeloid leukaemia (AML). Here, we report a human single-chain variable fragment (scFv), identified via yeast surface display, that specifically binds to the NPM1c epitope–HLA-A2 complex but not to HLA-A2 or to HLA-A2 loaded with control peptides. In vitro and in mice, CAR-T cells with the scFv exhibit potent cytotoxicity against NPM1c+HLA-A2+ leukaemia cells and primary AML blasts, but not NPM1c–HLA-A2+ leukaemia cells or HLA-A2– tumour cells. Therapies using NPM1c CAR-T cells for the treatment of NPM1c+HLA-A2+ AML may limit on-target–off-tumour toxicity and tumour resistance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the main findings of this study are available within the paper and its Supplementary Information. Raw data generated for this study are available in Figshare with the identifier https://doi.org/10.6084/m9.figshare.12922520 (ref. 47).
Change history
16 December 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41551-020-00677-7
References
Coulie, P. G., Van den Eynde, B. J., van der Bruggen, P. & Boon, T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat. Rev. Cancer 14, 135–146 (2014).
Srivastava, S. & Riddell, S. R. Chimeric antigen receptor T cell therapy: challenges to bench-to-bedside efficacy. J. Immunol. 200, 459–468 (2018).
Blankenstein, T., Leisegang, M., Uckert, W. & Schreiber, H. Targeting cancer-specific mutations by T cell receptor gene therapy. Curr. Opin. Immunol. 33, 112–119 (2015).
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015).
Van der Lee, D. I. et al. Mutated nucleophosmin 1 as immunotherapy target in acute myeloid leukemia. J. Clin. Invest. 129, 774–785 (2019).
Verdegaal, E. M. et al. Neoantigen landscape dynamics during human melanoma–T cell interactions. Nature 536, 91–95 (2016).
Thomas, D. & Majeti, R. Biology and relevance of human acute myeloid leukemia stem cells. Blood 129, 1577–1585 (2017).
Dombret, H. & Gardin, C. An update of current treatments for adult acute myeloid leukemia. Blood 127, 53–61 (2016).
Dohner, H., Weisdorf, D. J. & Bloomfield, C. D. Acute myeloid leukemia. N. Engl. J. Med. 373, 1136–1152 (2015).
Ossenkoppele, G. J., Janssen, J. J. & van de Loosdrecht, A. A. Risk factors for relapse after allogeneic transplantation in acute myeloid leukemia. Haematologica 101, 20–25 (2016).
Ley, T. J. et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 368, 2059–2074 (2013).
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013).
Kandoth, C. et al. Mutational landscape and significance across 12 major cancer types. Nature 502, 333–339 (2013).
Papaemmanuil, E. et al. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 374, 2209–2221 (2016).
Falini, B. et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N. Engl. J. Med. 352, 254–266 (2005).
Greiner, J. et al. Mutated regions of nucleophosmin 1 elicit both CD4+ and CD8+ T-cell responses in patients with acute myeloid leukemia. Blood 120, 1282–1289 (2012).
Greiner, J. et al. Immune responses against the mutated region of cytoplasmatic NPM1 might contribute to the favorable clinical outcome of AML patients with NPM1 mutations (NPM1mut). Blood 122, 1087–1088 (2013).
Chao, G. et al. Isolating and engineering human antibodies using yeast surface display. Nat. Protoc. 1, 755–768 (2006).
Choo, J. A., Liu, J., Toh, X., Grotenbreg, G. M. & Ren, E. C. The immunodominant influenza A virus M158–66 cytotoxic T lymphocyte epitope exhibits degenerate class I major histocompatibility complex restriction in humans. J. Virol. 88, 10613–10623 (2014).
Van Deventer, J. A., Kelly, R. L., Rajan, S., Wittrup, K. D. & Sidhu, S. S. A switchable yeast display/secretion system. Protein Eng. Des. Sel. 28, 317–325 (2015).
Quentmeier, H. et al. Cell line OCI/AML3 bears exon-12 NPM gene mutation-A and cytoplasmic expression of nucleophosmin. Leukemia 19, 1760–1767 (2005).
Lorente, E., Garcia, R. & Lopez, D. Allele-dependent processing pathways generate the endogenous human leukocyte antigen (HLA) class I peptide repertoire in transporters associated with antigen processing (TAP)-deficient cells. J. Biol. Chem. 286, 38054–38059 (2011).
Leskov, I. et al. Rapid generation of human B-cell lymphomas via combined expression of myc and Bcl2 and their use as a preclinical model for biological therapies. Oncogene 32, 1066–1072 (2013).
Matsueda, S. et al. Identification of prostate-specific G-protein coupled receptor as a tumor antigen recognized by CD8+ T cells for cancer immunotherapy. PLoS ONE 7, e45756 (2012).
Jennifer, B., Carl, H. J., Andreas, L., Marcela, M. & John, S. Treatment of cancer using humanized anti-CD19 chimeric antigen receptor. US patent 20140271635A1[P] (2014).
Hosken, N. A. & Bevan, M. J. Defective presentation of endogenous antigen by a cell line expressing class I molecules. Science 248, 367–370 (1990).
Bossi, G. et al. Examining the presentation of tumor-associated antigens on peptide-pulsed T2 cells. Oncoimmunology 2, e26840 (2013).
Pallasch, C. P. et al. Sensitizing protective tumor microenvironments to antibody-mediated therapy. Cell 156, 590–602 (2014).
Chen, Q., Khoury, M. & Chen, J. Expression of human cytokines dramatically improves reconstitution of specific human-blood lineage cells in humanized mice. Proc. Natl Acad. Sci. USA 106, 21783–21788 (2009).
Shah, N. N. & Fry, T. J. Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol. 16, 372–385 (2019).
Salmikangas, P., Kinsella, N. & Chamberlain, P. Chimeric antigen receptor T-cells (CAR T-cells) for cancer immunotherapy—moving target for industry? Pharm. Res. 35, 152 (2018).
Brudno, J. N. & Kochenderfer, J. N. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 34, 45–55 (2019).
Gill, S. et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood 123, 2343–2354 (2014).
Kenderian, S. S. et al. CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia 29, 1637–1647 (2015).
Uhlen, M. et al. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Watanabe, K. et al. Target antigen density governs the efficacy of anti-CD20-CD28-CD3 ζ chimeric antigen receptor-modified effector CD8+ T cells. J. Immunol. 194, 911–920 (2015).
Walker, A. J. et al. Tumor antigen and receptor densities regulate efficacy of a chimeric antigen receptor targeting anaplastic lymphoma kinase. Mol. Ther. 25, 2189–2201 (2017).
Dubrovsky, L. et al. T cell receptor mimic antibodies for cancer therapy. Oncoimmunology 5, e1049803 (2016).
Watanabe, K., Kuramitsu, S., Posey, A. J. & June, C. H. Expanding the therapeutic window for CAR T cell therapy in solid tumors: the knowns and unknowns of CAR T cell biology. Front. Immunol. 9, 2486 (2018).
Zhang, J. & Wang, L. The emerging world of TCR-T cell trials against cancer: a systematic review. Technol. Cancer Res. Treat. https://doi.org/10.1177%2F1533033819831068 (2019).
Morris, E. C. & Stauss, H. J. Optimizing T-cell receptor gene therapy for hematologic malignancies. Blood 127, 3305–3311 (2016).
Amir, A. L. et al. PRAME-specific Allo-HLA-restricted T cells with potent antitumor reactivity useful for therapeutic T-cell receptor gene transfer. Clin. Cancer Res. 17, 5615–5625 (2011).
Provasi, E. et al. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat. Med. 18, 807–815 (2012).
Bendle, G. M. et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat. Med. 16, 565–570 (2010).
Van Loenen, M. M. et al. Mixed T cell receptor dimers harbor potentially harmful neoreactivity. Proc. Natl Acad. Sci. USA 107, 10972–10977 (2010).
Matsui, K. et al. Low affinity interaction of peptide-MHC complexes with T cell receptors. Science 254, 1788–1791 (1991).
Xie, G. et al. Dataset for CAR-T cells targeting a nucleophosmin neoepitope exhibit potent specific activity in mouse models of acute myeloid leukaemia (Figshare, 2020); https://doi.org/10.6084/m9.figshare.12922520
Acknowledgements
We are grateful to K. D. Wittrup and A. W. Tisdale for the yeast display library and yeast display/secretion system. We thank B. Turner and S. A. Nordeen for assistance with the Octet biolayer interferometry experiment, V. Spanoudaki for assisting with mouse BLI and the Koch Institute Flow Cytometry Core for assistance. This work was supported in part by National Institutes of Health grant nos. AI69208, CA197605 and NS104315, the Ivan R. Cottrell Professorship and Research Fund, Koch Institute Support (core) Grant P30-CA14051 from the National Cancer Institute, National Institutes of Health Pre-Doctoral Training Grant T32GM007287 and a gift from C2Y Therapeutics.
Author information
Authors and Affiliations
Contributions
J.C. conceived of the idea and provided experimental advice and funding support. J.C. and G.X. designed the study. G.X. designed and performed the isolation of AIQ–HLA-A2-specific scFv using the yeast display scFv library. G.X., N.A.I. and Y. Li designed and generated the lentivirus vectors. G.X. and N.A.I. performed scFv–Fc protein production and characterization and determined scFV–Fc affinity by biolayer interferometry. G.X. and D.B. performed the CAR-T-cell production and characterization studies. B.J. and H.D. performed tissue preparation for flow cytometry analysis. G.X., B.J., Y. Li and H.D. performed the flow cytometry analysis. G.X. conducted the in vivo tumour studies and BLI. N.A.I. performed the western blot assay. G.X., H.D., Y. Liang and R.R. prepared and analysed the primary AML samples. G.X. prepared the figures and performed the statistical analyses. G.X. and J.C. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have filed a provisional patent application on the identified scFv and its applications.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–8 and references.
Rights and permissions
About this article
Cite this article
Xie, G., Ivica, N.A., Jia, B. et al. CAR-T cells targeting a nucleophosmin neoepitope exhibit potent specific activity in mouse models of acute myeloid leukaemia. Nat Biomed Eng 5, 399–413 (2021). https://doi.org/10.1038/s41551-020-00625-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-020-00625-5
This article is cited by
-
Challenges and strategies associated with CAR-T cell therapy in blood malignancies
Experimental Hematology & Oncology (2024)
-
Functional CRISPR screens in T cells reveal new opportunities for cancer immunotherapies
Molecular Cancer (2024)
-
Mutation-specific CAR T cells as precision therapy for IGLV3-21R110 expressing high-risk chronic lymphocytic leukemia
Nature Communications (2024)
-
Construction of a risk model and prediction of prognosis and immunotherapy based on cuproptosis-related LncRNAs in the urinary system pan-cancer
European Journal of Medical Research (2023)
-
Neoantigens: promising targets for cancer therapy
Signal Transduction and Targeted Therapy (2023)