Abstract
Herein, we studied the effects of the novel nonsteroidal selective mineralocorticoid receptor (MR) blocker, esaxerenone, on blood pressure and renal injury in Dahl salt-sensitive (DSS) rats. We also monitored intact urinary and total angiotensinogen (AGT). DSS rats were given a normal salt diet (NS: 0.4% NaCl, n = 10), a high-salt diet (HS: 8% NaCl, n = 10), HS + esaxerenone (1 mg/kg/day, p.o., n = 10), or HS + losartan (angiotensin II receptor blocker, 10 mg/kg/day, p.o., n = 10) for 6 weeks. Glomerular and tubulointerstitial tissues were obtained via a laser capture method. HS-treated DSS rats developed hypertension, albuminuria, and glomerular injury, which were associated with increased glomerular desmin staining and reduced mRNA levels of glomerular podocin and nephrin. HS-treated DSS rats also showed tubulointerstitial fibrosis with an increase in renal oxidative stress (4-hydroxynonenal staining). The urinary ((total AGT−intact AGT)/intact AGT) ratio, an indicator of intrarenal renin activity, was significantly suppressed in HS-treated DSS rats. Treatment with esaxerenone significantly decreased blood pressure, while losartan did not. Furthermore, esaxerenone attenuated the development of albuminuria, glomerular injury, and tubulointerstitial fibrosis more than losartan did, and this effect was associated with reduced renal oxidative stress. These data indicate that esaxerenone has antihypertensive and renal protective effects in salt-dependent hypertensive mice with suppressed intrarenal renin activity, as indicated by low levels of the urinary (total AGT−intact AGT)/intact AGT ratio.
Similar content being viewed by others
Introduction
Inappropriate activation of the mineralocorticoid receptor (MR) contributes to the development of salt-dependent hypertension and renal injury [1,2,3]. Recent studies have revealed that a high-salt (HS) diet induces salt-sensitive hypertension in Dahl salt-sensitive (DSS) rats in a ligand-independent manner through Rac-1-dependent MR activation [4]. Treatment with steroidal MR antagonists, such as spironolactone and eplerenone, effectively decreased blood pressure in DSS hypertensive rats [5,6,7], although their plasma aldosterone levels were very low [5, 6]. A double-blind crossover trial also revealed that in hypertensive patients who showed low renin and normal plasma potassium levels, spironolactone was a more effective natriuretic antihypertensive agent than amiloride, losartan, and thiazide [8]. These data indicate that MR is a potential therapeutic target for salt-dependent hypertension.
Because salt-dependent hypertension is a major risk factor for renal injury and cardiovascular disease [1, 9], it is important to clinically diagnose these patients. In this regard, subjects with salt-dependent hypertension usually show a suppressed renin–angiotensin system (RAS) due to reduced renin release from juxtaglomerular cells [10]. Consistently, HS diets lead to markedly decreased plasma angiotensin II levels, which are accompanied by reduced plasma renin activity (PRA) [10]. Furthermore, blockade of RAS with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) was less effective in decreasing blood pressure in low-renin DSS rats [11, 12] and in hypertensive patients who showed blood pressure with enhanced salt sensitivity [13,14,15]. However, diagnosis of salt-sensitive and RAS inhibitor-resistant hypertensive patients by testing the PRA is not common because of its circadian variation, instability, and impressionability [10]. Furthermore, a specific method for monitoring renin activity in the kidney is not available to date.
Recently, we developed a new method for stably evaluating renin activity by measuring total and intact angiotensinogen (AGT) with sandwich enzyme-linked immunosorbent assay (ELISA) [10, 16, 17]. In brief, the amount of des (angiotensin I) AGT can be estimated by (total AGT−intact AGT). Theoretically, the ratio of des (angiotensin I) AGT to intact AGT reflects how much intact AGT is cleaved to des (angiotensin I) AGT by renin [10]. In the present study, we examined whether the urinary ratio of (total AGT−intact AGT) to intact AGT, a potential stable indicator of intrarenal renin activity [10], is actually suppressed in low-renin DSS hypertensive rats. We also compared the antihypertensive and renoprotective effects of MR blockade with the newly developed nonsteroidal MR blocker, esaxerenone [18, 19], with RAS blockade of the ARB, losartan, in DSS rats.
Materials and methods
Materials
The selective nonsteroidal MR blocker, esaxerenone [(S)-1-(2-hydroxyethyl)-4-methyl-N-[4-(methylsulfonyl) phenyl]-5-[2-(trifluoromethyl) phenyl]-1H-pyrrole-3-carbox-amide], was provided by Daiichi Sankyo Co., Ltd (Tokyo, Japan) [18, 19].
Animals
All experimental procedures were performed according to the guidelines of the Care and Use Committee of Kagawa University. Four-week-old male DSS-Iwai rats (Japan SLC, Inc., Japan) weighing 200–220 g at the beginning of the experiments were fed a standard rat chow (0.5% NaCl) for 1 week. At 5 weeks of age, DSS rats were randomly selected to receive a normal salt diet (NS: 0.5% NaCl, n = 30) or an HS diet (8% NaCl, n = 30) for 6 weeks. At this age, the HS- and NS-fed DSS rats were also randomly divided into three groups as follows (10 rats in each group): (1) vehicle (0.5% carboxymethyl cellulose), (2) esaxerenone (1 mg/kg/day, p.o.), and (3) losartan (10 mg/kg/day, p.o.). The dosages of esaxerenone and losartan were determined based on previous studies on rats [19,20,21].
Blood pressure was measured by a noninvasive tail-cuff technique [6]. After a 15-min rest, systolic blood pressure (SBP) was measured at least five consecutive times on conscious rats by tail-cuff plethysmography (model BP-98A; Softron Co., Tokyo, Japan). The three lowest values were averaged. Twenty-four-hour urine sampling was performed using metabolic cages. Urine samples were stored at −30 °C after centrifugation.
Sample collection
At the end of the experiment, animals were anesthetized by isoflurane, and blood was quickly collected in EDTA-containing tubes via the abdominal aorta and cooled on ice. Then, animals were euthanized by an overdose of pentobarbital (250 mg/kg, i.p.), and the kidneys were harvested. Whole blood was centrifuged at 4 °C for 10 min to separate the plasma. The kidney tissues were cut and fixed in 10% buffered paraformaldehyde or embedded in Tissue-Tek OCT compound (Sakura Finetech, Tokyo, Japan). The remaining tissues were snap frozen in liquid nitrogen. Renal cortical tissues were also collected in RNAlater and kept at 4 °C overnight.
Histopathological examination
Kidneys were fixed in 10% formalin (pH 7.4), embedded in paraffin, cut into 3 μm sections, and mounted on slides. The sections were then stained with periodic acid-Schiff (PAS) or Azan reagent. Images were evaluated using light microscopy (BX-51/ DP-72; Olympus, Tokyo, Japan). PAS- and Azan-positive areas were determined using ImageJ software (National Institutes of Health, Bethesda, MD, USA) [12, 17].
Immunohistochemistry
Immunohistochemical staining of desmin [6] and 4-hydroxynonenal (4-HNE) [6] was performed with Histofine Simple Stain MAX-PO MULTI (Nichirei Biosciences, Tokyo, Japan). After deparaffinization with xylene, sections were incubated with 0.3% hydrogen peroxide for 15 min (for desmin) or 30 min (for 4-HNE) to block endogenous peroxidases. For desmin antigen retrieval, sections were incubated for 30 min in 0.01 mol/L citrate buffer (pH 6.0) at 100 °C. Proteinase K (DAKO Cytomation, Glostrup, Denmark) was used for 4-HNE antigen retrieval by incubation for 10 min. After blocking with 10% goat serum, sections were incubated overnight at 4 °C with primary antibodies, each at a 1:200 dilution (anti-human desmin rat monoclonal antibody, D33, DAKO Cytomation; anti-4-HNE antibody, ab46545, Abcam, Cambridge, UK). After washing sections and incubating them with secondary antibodies for 1 h at room temperature, DAB substrate (DAKO Cytomation) was used to visualize immunohistochemical staining. Finally, counterstaining was performed with hematoxylin (DAKO Cytomation). Positively stained areas were analyzed using ImageJ software.
Laser capture microdissection
Laser capture microdissection (LCM) was performed as previously described [6]. Frozen tissues embedded in OCT were cryosectioned into 10 μm sections and fast-stained using an Arcturus histogene frozen section staining kit (Ambion Inc., Austin, TX, USA). For each sample, 300–400 glomeruli were captured under direct visualization with CapSure HS LCM tubes using a laser microdissector pressure-catapulting device (Arcturus® LCM; Applied Biosystems, Waltham, MA, USA). From the remaining tissues, we also collected tubulointerstitial tissues. mRNA was extracted from glomerular and tubulointerstitial tissues using an RNAqueous-Micro kit (Ambion Inc.).
Real time reverse transcriptase PCR
We obtained glomerular and renal interstitial tissues by the LCM technique. The mRNA levels of β-actin, transforming growth factor-β (TGF-β), type 1 collagen, gp47phox, p22pho, podocin, and nephrin were analyzed by real-time PCR using a 7300 Fast Real-Time PCR System (Applied Biosystems, Foster City, USA) and a Light Cycler Fast Start DNA Master SYBR Green I kit (Applied Biosystems). The rat primer sequences (forward and reverse) used were β-actin, 5′-CCCTGGCTCCTAGCACCAT-3′, 5′- CCTGCTTGCTGATCCACATCT-3′; TGF-β, 5′-GCCTGAGTGGCTGTCTTTTGA-3′, 5′-GAAGCGAAAGCCCTGTATTCC-3′; type 1 collagen, 5′-TCACCTACAGCACGCTTG-3′, 5′-GGTCTGTTTCCAGGGTTG-3′; gp47phox, 5′-GGATCACAGAAGGTCCCTAGC-3′, 5′-AGAAGTTCAGGGCGTTCACC-3′; p22phox, 5′-TGGCCTGATCCTCATCACAG-3′, 5′-AGGCACGGACAGCAGTAAGT-3′; podocin, 5′-CCTTTCCATGAGGTGGTAACCA-3′, 5′-GGATGGCTTTGGA-3′; nephrin, 5′-GTTCAGCTGGGAGAGACTGG-3′, and 5′-AATCGGACGACAAGACGAAC-3′. Relative mRNA levels were determined using the 2−ΔΔCt method. The ΔΔCt value was calculated using data from the NS + vehicle group.
Urinary AGT
The intact and total urinary AGT concentrations were determined using a rat AGT ELISA kit (IBL Co., Ltd, Fujioka, Japan) as previously described [17].
Other parameters
Urinary albumin and protein levels were measured using commercially available kits (Rat Albumin ELISA Kit, Shibayagi, Gunma, Japan; MicroTP-test, Wako Co., Ltd, Osaka, Japan, respectively). PRA and plasma aldosterone concentrations were measured by radioimmunoassay as previously described [5, 6, 22]. Blood urea nitrogen (BUN) concentrations were measured using a BUN assay kit (Wako Co., Ltd).
Statistical analysis
Data are presented as the means ± SEM. One-way or two-way analysis of variance followed by Bonferroni post hoc test was used. P < 0.05 was considered statistically significant.
Results
Blood pressure, body weight, and renal function
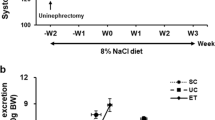
The HS diet significantly increased the SBP (from 113 ± 2 to 236 ± 8 mmHg) of DSS rats, whereas the NS diet only exhibited a slight SBP elevation (Fig. 1). Concomitant treatment with esaxerenone significantly suppressed the HS-induced SBP elevation (203 ± 4 mmHg), while losartan did not significantly change the SBP (232 ± 4 mmHg).
At 11 weeks of age, HS-treated rats showed a marked elevation in urinary volume compared with the NS-treated rats (Supplementary Table 1). Treatment with losartan or esaxerenone did not change urinary volume but significantly decreased the plasma BUN levels in the HS-fed DSS rats (Supplementary Table 1). The HS diet significantly increased urinary protein and albumin excretions in DSS rats. This effect was significantly suppressed by treatment with losartan or esaxerenone. However, esaxerenone had a significantly stronger effect than losartan (Supplementary Table 1).
PRA and plasma aldosterone concentration
As shown in Supplementary Table 1, the HS diet significantly decreased both the PRA and the plasma aldosterone concentration in DSS rats. Treatment with losartan or esaxerenone significantly increased the PRA in both the NS- and HS-fed DSS rats. However, the plasma aldosterone levels were significantly decreased by losartan and increased by esaxerenone in NS-fed DSS rats. In the HS-fed DSS rats, the plasma aldosterone levels tended to be decreased by losartan and increased by esaxerenone. However, these changes were not statistically significant.
Urinary AGT excretion
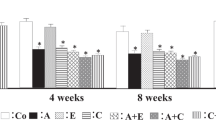
In the NS-fed DSS rats, concomitant treatment with losartan or esaxerenone did not significantly change the total urinary AGT or intact urinary AGT excretion (Fig. 2a, b). We also determined the amount of des (angiotensin I) AGT, which was calculated as (total AGT−intact AGT). The ratio of (total AGT−intact AGT) to intact AGT in the urine theoretically reflects how much intact AGT is cleaved by renin to des (angiotensin I) AGT in the kidney [10]. The results showed that neither losartan nor esaxerenone changed the ratio of (total AGT−intact AGT) to intact AGT in the NS-fed DSS rats (Fig. 2c). However, the HS-fed DSS rats showed markedly greater total urinary AGT excretion (16,968 ± 3,943 ng/day) compared with the NS-fed rats (306 ± 35 ng/day). Similarly, the intact urinary AGT excretion was significantly increased in the HS-fed DSS rats (6479 ± 1283 vs. 36 ± 9 ng/day in the NS-fed DSS rats). In the HS-fed DSS rats, treatment with esaxerenone significantly decreased the total urinary AGT (from 16,968 ± 3943 to 4164 ± 1798 ng/day) and the intact AGT excretion (from 6479 ± 1283 to 2209 ± 627 ng/day), whereas losartan did not (Fig. 2a, b). Interestingly, the HS-fed DSS rats showed a markedly reduced (total AGT−intact AGT) to intact AGT ratio in urine (1.3 ± 0.1%) compared with the NS-fed DSS rats (10.0 ± 1.8%; Fig. 2c), suggesting suppression of renin activity in the kidney. The reduced urinary ratio of des (angiotensin I) AGT to intact AGT was affected by neither losartan nor esaxerenone.
Glomerular and tubulointerstitial histopathological changes
In DSS rats, the HS diet induced severe glomerular injury, as assessed by an increase in the PAS-positive areas in the glomeruli (Fig. 3a). Treatment with losartan tended to attenuate the HS-induced glomerular injury. However, these changes were not statistically significant. In contrast, esaxerenone treatment substantially reduced the glomerular PAS-positive area in DSS rats (Fig. 3a, b).
Glomerular podocyte injury was determined by desmin staining with immunohistochemistry [6]. The HS-fed DSS rats showed an increased glomerular desmin-positive area (Fig. 4a, b). In the HS-fed DSS rats, treatment with losartan slightly but significantly decreased the glomerular desmin-positive area. We also measured the gene expression of podocin and nephrin, which are components of the slit diaphragm between adjacent podocytes in glomeruli [6]. Losartan tended to increase the glomerular podocin and nephrin mRNA levels, but these changes were not statistically significant (Fig. 4c, d). However, treatment with esaxerenone significantly decreased the glomerular desmin-positive area and increased the glomerular mRNA levels of podocin and nephrin in HS-fed DSS rats (Fig. 4a–d).
Glomerular podocyte injury in DSS rats. a Representative micrographs of desmin-stained renal sections. Original magnification, ×200. b Quantitative analysis of the desmin-positive area in the glomerulus. c mRNA expression of podocin. d mRNA expression of nephrin. The ΔΔCt value was calculated using data from the NS + vehicle group. *P < 0.05 vs. NS + vehicle. #P < 0.05, HS + vehicle vs. HS + losartan or HS + esaxerenone. †P < 0.05, HS + losartan vs. HS + esaxerenone
In DSS rats, the HS diet caused significant tubulointerstitial fibrosis, as assessed by quantification of the Azan-positive area in the renal interstitium (Fig. 5a, b). The HS-induced tubulointerstitial fibrosis was attenuated by esaxerenone but not by losartan in HS-fed DSS (Fig. 5a, b). Similarly, a significant reduction in the tubulointerstitial tissue TGF-β and type 1 collagen mRNA levels was induced by treatment with esaxerenone but not with losartan (Fig. 5c, d).
Tubulointerstitial fibrosis in DSS rats. a Representative images of Azan-stained renal sections. Original magnification, ×200. b Quantitative analysis of the Azan-positive area. c mRNA expression of transforming growth factor β (TGF-β). d mRNA expression of type 1 collagen (collagen-1). The ΔΔCt value was calculated using data from the NS + vehicle group. *P < 0.05 vs. NS + vehicle. #P < 0.05, HS + vehicle vs. HS + esaxerenone. †P < 0.05, HS + losartan vs. HS + esaxerenone
Oxidative stress
Tubulointerstitial oxidative stress was evaluated by 4-HNE immunohistochemistry [6]. The HS-fed DSS rats showed a significantly increased 4-HNE-positive area in the renal interstitium (Fig. 6a, b) and increased mRNA levels of the NADPH oxidase subunits, p22phox and gp47phox, in tubulointerstitial tissues (Fig. 6c, d). The tubulointerstitial 4-HNE-positive area was significantly decreased by losartan and esaxerenone in HS-fed DSS rats (Fig. 6a, b). In these animals, losartan significantly decreased the p22phox mRNA levels in tubulointerstitial tissues, whereas esaxerenone treatment significantly decreased both p22phox and gp47phox mRNA levels (Fig. 6c, d).
Renal oxidative stress in DSS rats. a Representative micrographs of 4-hydroxynonenal (4-HNE)-stained renal sections. Original magnification, ×200. b Quantitative analysis of the 4-HNE-positive area in the renal interstitium. c mRNA expression of p22phox. d mRNA expression of gp47phox. The ΔΔCt value was calculated using data from the NS + vehicle group. *P < 0.05 vs. NS + vehicle. #P < 0.05, HS + vehicle vs. HS + losartan or HS + esaxerenone. †P < 0.05, HS + losartan vs. HS + esaxerenone
Discussion
There is substantial evidence that low-renin salt-dependent hypertension is resistant to RAS blockade with ACEIs and ARBs [11,12,13,14,15], whereas treatment with MR antagonists effectively decreases blood pressure in these patients [1,2,3,4,5,6,7]. However, it is difficult to clinically diagnosis low-renin salt-dependent hypertension. Furthermore, it is not yet possible to monitor intrarenal renin activity. Here, we demonstrated for the first time that HS-treated DSS rats showed a markedly reduced urinary (total AGT−intact AGT)/intact AGT ratio, suggesting suppressed intrarenal renin activity. In agreement with previous studies [11], losartan, an ARB, did not substantially decrease blood pressure in low-renin DSS hypertensive rats. In contrast, we showed that treatment with the nonsteroidal MR blocker, esaxerenone, resulted in effective blood pressure reduction in these animals. These data indicate that MR blockade with esaxerenone has antihypertensive and renoprotective effects during the development of low-renin salt-dependent hypertension, as indicated by the urinary (total AGT−intact AGT)/intact AGT ratio.
In subjects with low-renin salt-dependent hypertension, not only plasma renin and angiotensin II but also plasma aldosterone levels are usually reduced [5, 10]. However, intrarenal MR was activated by HS in a ligand-independent manner through Rac1-mediated pathways, thereby contributing to the development of salt-dependent hypertension [4, 23]. Consistently, treatment with steroidal MR antagonists significantly decreased blood pressure in DSS rats, even though the plasma aldosterone levels were significantly reduced [4,5,6,7]. Recently, we have established a method for stably evaluating renin activity by measuring intact AGT and des (angiotensin I) AGT (calculated as total AGT−intact AGT) [16, 17]. Namely, the ratio of des (angiotensin I) AGT to intact AGT theoretically reflects how much intact AGT is cleaved by renin [10]. The present study showed that the ratio of des (angiotensin I) AGT to intact AGT in the urine was approximately 90% reduced by HS in DSS rats. These data indicate that the ratio of des (angiotensin I) AGT to intact AGT in the urine is a potential biomarker for identifying low-renin salt-sensitive hypertension. We also observed that neither esaxerenone nor losartan significantly changed the ratio of urinary des (angiotensin I) AGT to intact AGT. These data suggest that during the development of HS-induced hypertension, suppressed intrarenal renin activity is not affected by treatment with esaxerenone or losartan.
A growing body of evidence has indicated that inappropriate activation of the aldosterone/MR signaling pathway induces glomerular injury [1, 3]. In vitro studies have shown that activation of this pathway induces mesangial cell proliferation [24, 25] and podocyte injury [6, 23]. In agreement with earlier studies that used steroidal MR antagonists, the present study showed that treatment with the nonsteroidal MR blocker [6, 26, 27], esaxerenone, reduced blood pressure and protected against glomerular injury (glomerular PAS-positive area), particularly albuminuria and podocyte injury (glomerular desmin-positive area) in DSS hypertensive rats. We also confirmed that the mRNA expression of essential podocyte components, such as podocin and nephrin, in glomerular tissues was markedly increased by treatment with esaxerenone. However, treatment with losartan did not change blood pressure and glomerular injury, though it partially attenuated podocyte injury. The glomerular podocin and nephrin mRNA expression also tended to be increased by losartan, but these results were not statistically significant. Similarly, in agreement with a previous study [20], we demonstrated that tubulointerstitial fibrosis was significantly attenuated by treatment with esaxerenone in DSS rats. Our data also showed that the esaxerenone-induced attenuation of renal interstitial fibrosis was associated with downregulated tubulointerstitial tissue TGF-β and type 1 collagen mRNA expression. As blood pressure was significantly decreased by esaxerenone treatment, part of the renoprotective effects of esaxerenone against glomerular injury and tubulointerstitial fibrosis may be mediated by blood pressure reduction.
Both angiotensin II and aldosterone/MR stimulate oxidative stress through activation of NADPH oxidase-dependent superoxide anion production [28,29,30]. Several studies have also indicated that NADPH oxidase and oxidative stress play a critical role in the pathogenesis of hypertension and renal injury in DSS rats [6, 31, 32]. The present study showed that both losartan and esaxerenone suppressed the renal interstitial oxidative stress marker, 4-NHE, and the expression of the tubulointerstitial tissue NADPH oxidase component, p22phox. However, tubulointerstitial tissue gp47phox was significantly decreased by esaxerenone, but not by losartan. These data suggest that treatment with esaxerenone has strong renal antioxidative effects during the development of salt-induced hypertension. Recently, Lattenist et al. [33] showed that treatment with another nonsteroidal MR antagonist, finerenone, prevented the transition from acute kidney injury to chronic kidney disease, which was associated with reduced expression of oxidative stress markers, such as malondialdehyde, in renal tissues and decreased plasma 8-hydroxyguanosine concentrations. Similarly, in obese (fa/fa) Zucker rats with metabolic syndrome, the beneficial effects of finerenone against left ventricular dysfunction and proteinuria were associated with decreased myocardial oxidative stress [34]. As increased bioavailability of nitric oxide was also observed, it can be speculated that superoxide anion production would be reduced by finerenone treatment [34]. Taken together, these data combined with the present results support the concept that nonsteroidal MR blockers protect renal tissue by suppressing renal oxidative stress.
In the present study, we did not evaluate the difference in the beneficial effect observed between the nonsteroidal MR antagonist, esaxerenone, and steroidal MR antagonists (spironolactone or eplerenone) in DSS hypertensive rats. However, previous pharmacokinetics studies have shown that esaxerenone has higher selectivity and greater binding affinity against MR than spironolactone and eplerenone, respectively [18,19,20]. Interestingly, recent clinical studies reported that nonsteroidal MR antagonists conferred a lower risk of hyperkalemia [35,36,37]. Further clinical studies are needed to confirm the beneficial effect of esaxerenone in patients with salt-dependent hypertension. We also did not examine the combined effect of losartan and esaxerenone on renal injury in DSS hypertensive rats. Previous clinical studies have shown that adding traditional steroidal MR antagonists, such as spironolactone or eplerenone, to ACEIs or ARBs yielded significant reductions in albuminuria in patients with CKD [1, 2]. We previously showed that the additive antiproteinuric effect of eplerenone with an ARB was associated with glomerular podocyte protection in type 2 diabetic rats [38]. However, clinical studies have also indicated a risk of hyperkalemia from treatment with traditional steroidal MR antagonists and ACEIs/ARBs in patients with advanced nephropathy [1, 39]. In this regard, a phase III clinical trial (ESAX-DN study) is ongoing to examine the safety and effectiveness of esaxerenone in patients with type 2 diabetic nephropathy who are treated with an ACEI or ARB (https://www.daiichisankyo.com/media_investors/media_relations/press_releases/detail/006726.html).
In conclusion, the present study suggests that the novel nonsteroidal MR blocker, esaxerenone, had antihypertensive and renal protective effects in mice with low-renin salt-dependent hypertension through its antioxidative effects. These data support the hypothesis that nonsteroidal MR blockade is a novel therapeutic strategy for salt-sensitive hypertension and associated renal disease.
References
Shibata S, Ishizawa K, Uchida S. Mineralocorticoid receptor as a therapeutic target in chronic kidney disease and hypertension. Hypertens Res. 2017;40:221–5.
Sato A. The necessity and effectiveness of mineralocorticoid receptor antagonist in the treatment of diabetic nephropathy. Hypertens Res. 2015;38:367–74.
Ueda K, Nagase M. Mineralocorticoid receptor activation as an etiological factor in kidney diseases. Clin Exp Nephrol. 2014;18:16–23.
Shibata S, Mu S, Kawarazaki H, Muraoka K, Ishizawa K, Yoshida S, et al. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest. 2011;121:3233–43.
Zhu A, Yoneda T, Demura M, Karashima S, Usukura M, Yamagishi M, et al. Effect of mineralocorticoid receptor blockade on the renal renin-angiotensin system in Dahl salt-sensitive hypertensive rats. J Hypertens. 2009;27:800–5.
Kitada K, Nakano D, Liu Y, Fujisawa Y, Hitomi H, Shibayama Y, et al. Oxidative stress-induced glomerular mineralocorticoid receptor activation limits the benefit of salt reduction in Dahl salt-sensitive rats. PLoS ONE. 2012;7:e41896.
Buss SJ, Backs J, Kreusser MM, Hardt SE, Maser-Gluth C, Katus HA, et al. Spironolactone preserves cardiac norepinephrine reuptake in salt-sensitive Dahl rats. Endocrinology. 2006;147:2526–34.
Hood SJ, Taylor KP, Ashby MJ, Brown MJ. The spironolactone, amiloride, losartan, and thiazide (SALT) double-blind crossover trial in patients with low-renin hypertension and elevated aldosterone-renin ratio. Circulation. 2007;116:268–75.
Kimura G, Dohi Y, Fukuda M. Salt sensitivity and circadian rhythm of blood pressure: the keys to connect CKD with cardiovascular events. Hypertens Res. 2010;33:515–20.
Nishiyama A, Kobori H. Independent regulation of renin-angiotensin-aldosterone system in the kidney. Clin Exp Nephrol. 2018;22:1231-9.
Takahashi H, Nakagawa S, Wu Y, Kawabata Y, Numabe A, Yanagi Y, et al. A high-salt diet enhances leukocyte adhesion in association with kidney injury in young dahl salt-sensitive rats. Hypertens Res. 2017;40:912–20.
Guo P, Nishiyama A, Rahman M, Nagai Y, Noma T, Namba T, et al. Contribution of reactive oxygen species to the pathogenesis of left ventricular failure in Dahl salt-sensitive hypertensive rats: effects of angiotensin II blockade. J Hypertens. 2006;24:1097–104.
Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M. et al. Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res. 2014;37:253–390.
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–20.
Lindhorst J, Alexander N, Blignaut J, Rayner B. Differences in hypertension between blacks and whites: an overview. Cardiovasc J Afr. 2007;18:241–47.
Yoshimoto T, Furuki T, Kobori H, Miyakawa M, Imachi H, Murao K, et al. Effects of sodium-glucose cotransporter 2 inhibitors on urinary excretion of intact and total angiotensinogen in patients with type 2 diabetes. J Investig Med. 2017;65:1057–61.
Li L, Konishi Y, Morikawa T, Zhang Y, Kitabayashi C, Kobara H, et al. Effect of a SGLT2 inhibitor on the systemic and intrarenal renin-angiotensin system in subtotally nephrectomized rats. J Pharmacol Sci. 2018;137:220–3.
Yamada M, Takei M, Suzuki E, Takakusa H, Kotsuma M, Washio T. et al. Pharmacokinetics, distribution, and disposition of esaxerenone, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist, in rats and monkeys. Xenobiotica. 2017;47:1090–103.
Arai K, Homma T, Morikawa Y, Ubukata N, Tsuruoka H, Homma T. Pharmacological profile of CS-3150, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist. Eur J Pharmacol. 2015;761:226–34.
Arai K, Tsuruoka H, Homma T. CS-3150, a novel non-steroidal mineralocorticoid receptor antagonist, prevents hypertension and cardiorenal injury in Dahl salt-sensitive hypertensive rats. Eur J Pharmacol. 2015;769:266–73.
Karanovic D, Grujic-Milanovic J, Miloradovic Z, Ivanov M, Jovovic D, Vajic UJ, et al. Effects of single and combined Losartan and Tempol treatments on oxidative stress, kidney structure and function in spontaneously hypertensive rats with early course of proteinuric nephropathy. PLoS ONE. 2016;11:e0161706.
Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid angiotensin I and angiotensin II concentrations during local angiotensin-converting enzyme inhibition. J Am Soc Nephrol. 2002;13:2207–12.
Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14:1370–76.
Nishiyama A, Yao L, Fan Y, Kyaw M, Kataoka N, Hashimoto K, et al. Involvement of aldosterone and mineralocorticoid receptors in rat mesangial cell proliferation and deformability. Hypertension. 2005;45:710–6.
Terada Y, Kobayashi T, Kuwana H, Tanaka H, Inoshita S, Kuwahara M, et al. Aldosterone stimulates proliferation of mesangial cells by activating mitogen-activated protein kinase 1/2, cyclin D1, and cyclin A. J Am Soc Nephrol. 2005;16:2296–305.
Kawarazaki H, Ando K, Nagae A, Fujita M, Matsui H, Fujita T. Mineralocorticoid receptor activation contributes to salt-induced hypertension and renal injury in prepubertal Dahl salt-sensitive rats. Nephrol Dial Transplant. 2010;25:2879–89.
Nagase M, Shibata S, Yoshida S, Nagase T, Gotoda T, Fujita T. Podocyte injury underlies the glomerulopathy of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension. 2006;47:1084–93.
Hitomi H, Kiyomoto H, Nishiyama A. Angiotensin II and oxidative stress. Curr Opin Cardiol. 2007;22:311–5.
Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, et al. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension. 2004;43:841–8.
Miyata K, Rahman M, Shokoji T, Nagai Y, Zhang GX, Sun GP, et al. Aldosterone stimulates reactive oxygen species production through activation of NADPH oxidase in rat mesangial cells. J Am Soc Nephrol. 2005;16:2906–12.
Nishiyama A, Yoshizumi M, Hitomi H, Kagami S, Kondo S, Miyatake A, et al. The SOD mimetic tempol ameliorates glomerular injury and reduces mitogen-activated protein kinase activity in Dahl salt-sensitive rats. J Am Soc Nephrol. 2004;15:306–15.
Onozato ML, Tojo A, Kobayashi N, Goto A, Matsuoka H, Fujita T. Dual blockade of aldosterone and angiotensin II additively suppresses TGF-beta and NADPH oxidase in the hypertensive kidney. Nephrol Dial Transplant. 2007;22:1314–22.
Lattenist L, Lechner SM, Messaoudi S, Le Mercier A, El Moghrabi S, Prince S, et al. Nonsteroidal mineralocorticoid receptor antagonist finerenone protects against acute kidney injury-mediated chronic kidney disease: role of oxidative stress. Hypertension. 2017;69:870–8.
Lachaux M, Barrera-Chimal J, Nicol L, Rémy-Jouet I, Renet S, Dumesnil A, et al. Short- and long-term administration of the non-steroidal mineralocorticoid receptor antagonist finerenone opposes metabolic syndrome-related cardio-renal dysfunction. Diabetes Obes Metab. 2018;20:2399–407.
Kato M, Furuie H, Shimizu T, Miyazaki A, Kobayashi F, Ishizuka H. Single- and multiple-dose escalation study to assess pharmacokinetics, pharmacodynamics and safety of oral esaxerenone in healthy Japanese subjects. Br J Clin Pharmacol. 2018;84:1821–9.
Pei H, Wang W, Zhao D, Wang L, Su GH, Zhao Z. The use of a novel non-steroidal mineralocorticoid receptor antagonist finerenone for the treatment of chronic heart failure: a systematic review and meta-analysis. Medicine (Baltimore). 2018;97:e0254.
Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. 2013;34:2453–63.
Nishiyama A, Kobori H, Konishi Y, Morikawa T, Maeda I, Okumura M, et al. Mineralocorticoid receptor blockade enhances the antiproteinuric effect of an angiotensin II blocker through inhibiting podocyte injury in type 2 diabetic rats. J Pharmacol Exp Ther. 2010;332:1072–80.
Nishiyama A. Pathophysiological mechanisms of mineralocorticoid receptor-dependent cardiovascular and chronic kidney disease. Hypertens Res. 2018 (in press).
Acknowledgements
We thank Michal Bell, PhD, from Edanz Group (www.edanzediting.com/ac) for editing the English text of a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was in part a collaborative study with Daiichi-Sankyo Co., Ltd (to AN). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. This study was also supported in part by the Salt Sciences Foundation (to AN).
Supplementary information
Rights and permissions
About this article
Cite this article
Li, L., Guan, Y., Kobori, H. et al. Effects of the novel nonsteroidal mineralocorticoid receptor blocker, esaxerenone (CS-3150), on blood pressure and urinary angiotensinogen in low-renin Dahl salt-sensitive hypertensive rats. Hypertens Res 42, 769–778 (2019). https://doi.org/10.1038/s41440-018-0187-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0187-1
Keywords
This article is cited by
-
Exploratory study on the relationship between urinary sodium/potassium ratio, salt intake, and the antihypertensive effect of esaxerenone: the ENaK Study
Hypertension Research (2024)
-
The renoprotective effect of esaxerenone independent of blood pressure lowering: a post hoc mediation analysis of the ESAX-DN trial
Hypertension Research (2023)
-
Upregulation of Piezo2 in the mesangial, renin, and perivascular mesenchymal cells of the kidney of Dahl salt-sensitive hypertensive rats and its reversal by esaxerenone
Hypertension Research (2023)
-
Antihypertensive Effect of Long-Term Monotherapy with Esaxerenone in Patients with Essential Hypertension: Relationship Between Baseline Urinary Sodium Excretion and Its Antihypertensive Effect
Advances in Therapy (2022)
-
Clinical Pharmacokinetics and Pharmacodynamics of Esaxerenone, a Novel Mineralocorticoid Receptor Antagonist: A Review
European Journal of Drug Metabolism and Pharmacokinetics (2022)