Abstract
The optimal level of sodium intake remains controversial, and the effects on a broad range of cardiovascular (CV) conditions remain unknown. The Evaluation of sodium intake for the prediction of cardiovascular events in Japanese high-risk patients (ESPRIT) is a prospective observational study designed to investigate whether sodium intake assessed by spot urine testing is associated with adverse CV events. A total of 520 patients who visited our cardiology clinic with various cardiovascular risk profiles were included. Sodium intake was estimated by spot urine testing at the time of entry, and the measurement was repeated at least every 6 months during follow-up. The primary endpoint was composed of (1) hospitalization due to heart failure, (2) acute coronary syndrome, (3) cerebrovascular events, and (4) documented CV deaths. The secondary endpoint was all-cause mortality. During the median follow-up period of 5.2 years, there were 105 composite CV events (3.9%/year), including 60 hospitalizations due to heart failure, 9 acute coronary syndromes, 21 cerebrovascular events, 15 CV deaths, and 26 cases of all-cause mortality. The average sodium excretion (from a median of 14 measurements) during the follow-up period was 3.52 ± 0.67 g/day. After adjustment for age, sex, and body weight, higher sodium excretion ( ≥ 4.0 g/day) was associated with composite CV events (hazard ratio 1.79, confidence interval 1.01–3.15 compared with the reference value of 3.0–3.49 g/day) but not all-cause mortality. The ESPRIT study showed that high sodium excretion (≥ 4.0 g/day) was associated with the predefined composite CV events (UMIN ID: UMIN000005419).
Similar content being viewed by others
Introduction
Despite numerous observational studies and clinical trials, the optimal level of sodium intake remains unclear. Recently, the Prospective Urban Rural Epidemiology (PURE) study [1] showed that sodium intake > 6 g/day was associated with a higher risk of increasing blood pressure, whereas only modest effects were seen at sodium intake levels between 3 and 6 g/day. In the Dietary Approaches to Stop Hypertension (DASH) trial [2], lower sodium intake (less than 2.5 g/day) significantly reduced blood pressure, although its relationship with long-term morbidity and mortality risk was unclear.
On the other hand, the competing activation of the renin–angiotensin–aldosterone system (RAAS) has been a concern [3]. The Trial of Hypertension Prevention (TOHP) study [4] showed that the effect between sodium excretion and outcomes is linear. However, within the PURE study, lower estimated sodium excretion was associated with a higher risk for cardiovascular (CV) events. Therefore, the optimal level of sodium remains unclear. To balance the potential risk of RAAS activation at low sodium intake levels with elevated blood pressure at high sodium intake levels, some researchers have advocated a goal for sodium intake of 3–4 g/day [5].
The Evaluation of sodium intake for the prediction of cardiovascular events in Japanese high-risk patients (ESPRIT) is a prospective, observational study designed to elucidate whether sodium intake evaluated by spot urine testing is associated with CV events. In this study, we used an average of samples collected during the observational periods because sodium intake varies from day to day and a single measurement at baseline might lead to erroneous conclusions [6,7,8].
Methods
Patients
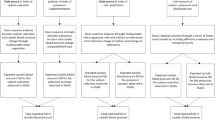
This was a single-center, prospective, observational study. Eligible patients included in this study were those who visited our cardiology clinic with at least one of the following CV conditions: (1) stable and compensated congestive heart failure, (2) reduced left ventricular ejection fraction ( < 50%), (3) brain natriuretic peptide (BNP) ≥ 100 pg/mL for any reason, (4) documented coronary artery disease, (5) cerebrovascular disease, (6) chronic kidney disease (CKD), estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2, and (7) and atrial fibrillation. Patients who had experienced CV events during the previous 6 months were excluded. Figure 1 shows the flow diagram of this study. During the entry period, 720 outpatients were screened to evaluate sodium intake by spot urine testing, but 200 patients did not meet the entry criteria. Thus, 520 patients were finally enrolled from April 2011 to March 2015 and followed until March 2017. Sodium intake was estimated by spot urine testing at the time of entry, and the measurement was repeated at least every 6 months during the follow-up period. In this study, average sodium excretions at the time of entry to the last follow-up were used for the subsequent analyses unless otherwise stated. The attending physicians explained the individual data to the patients and encouraged them to reduce their sodium intake through simple counseling. Furthermore, dietary counseling by expert dieticians was performed when necessary at the discretion of the attending physicians. During a median follow-up period of 5.2 years, five patients were lost to follow-up because of moving (three patients), alcoholism (one patient), or refusal to follow-up (one patient). However, the shortest follow-up duration was 868 days in these five patients, and their urine samples were measured ≥ 7 times; thus, they were all included in this study. Collection of urine samples was terminated in 46 patients during follow-up because they were followed by other hospitals/clinics (n = 33), admitted to the nursing home (n = 5), did not have an office visit (followed by telephone) (n = 6), and needed hemodialysis (n = 2), but follow-up was completed in all the patients until March 2017 (Fig. 1). Urine samples had been measured ≥ 4 times (median 12 times) in these patients.
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional ethics committee of the Ueki hospital, and informed written consent was obtained from all of the patients.
Endpoints
The primary endpoint of this study was composed of (1) hospitalization due to heart failure, (2) acute coronary syndrome, (3) cerebrovascular events, and (4) CV deaths. The secondary endpoint was all-cause mortality.
Definition
Hypertension was defined as either systolic blood pressure exceeding 140 mmHg or diastolic blood pressure exceeding 90 mmHg (office measurement of blood pressure) or taking antihypertensive medications. Diabetes mellitus was defined as fasting plasma glucose ≥ 126 mg/dL, casual glucose ≥ 200 mg/dL, HgA1c ≥ 6.5%, or using oral hypoglycemic medications or insulin. The left ventricular ejection fraction was calculated using echocardiography (Simpson’s method). The eGFR was calculated according to the Japanese version of the Modification of Diet in Renal Disease Study equation [9]. Coronary artery disease was diagnosed by cardiac catheterization (with acetylcholine or ergonovine provocative test when necessary) or typical angina symptoms with positive exercise test or relief of symptoms with sublingual nitroglycerin, or by history of myocardial infarction. Congestive heart failure (CHF) was defined as having New York Heart Association Functional Classification ≥ II symptoms and/or congestive signs requiring loop diuretics at the time of entry, but in this study, a history of CHF that required continued medical treatment was also included as having CHF. Hospitalization due to heart failure was defined as acute decompensated heart failure (ADHF) admission that required intravenous diuretic treatment. This was judged by the consensus of the first and second authors. CV death due to heart failure was defined as death in patients who were not hospitalized for ADHF, such as patients with hip fracture or pneumonia, but who subsequently died due to worsening heart failure. Cerebrovascular events and aortic dissection were confirmed by computed tomography and/or magnetic resonance imaging. Sudden deaths of unknown causes (within 24 h) were included in CV deaths.
Estimation of salt excretion
The daily salt excretion was estimated using the following equation [10, 11]:
Estimated 24-h urinary salt excretion (g/day) = 1.285 × (Na (mEq/L)/Cr (mg/dL) in spot urine testing x expected 24-h Cr excretion)0.392, where the expected 24-h Cr excretion (mg/day) = −2.04 × age (y/o) + 14.89 × weight (kg) + 16.14 × height (cm)−2244.45.
In this study, sodium excretion values were calculated from salt excretions multiplied by 0.3933. The spot urine was collected at the time of the office visits, usually between 9:00 am and 11:00 am. The BNP level was measured by an automated enzyme immunoassay analyzer, AIA-360 (TOSOH, Tokyo, Japan, detection limits, 5 pg/mL).
Statistical analysis
Data are presented as the mean ± standard deviation (SD), median (interquartile range, IQR), or percentage, as appropriate. Event frequencies were compared using the chi-squared test. Other comparisons between two groups of data were made with Student’s t test or the Mann–Whitney U test, as appropriate. When comparing ≥ 3 groups, analysis of variance or the Kruskal–Wallis test was used. We divided the patients into four groups ( < 3.0 g/day, 3.0–3.49 g/day, 3.5–3.99 g/day, and ≥ 4.0 g/day) for practical reasons. The outcomes are displayed with Kaplan–Meier event-free curves and were compared with the use of log-rank tests. The prognostic values of sodium excretion and clinical variables were analyzed using Cox proportional hazard models, and hazard ratios (HRs) are described with their 95% confidence intervals (CIs). A P-value of < 0.05 was accepted as statistically significant. The statistical software package JMP (version 11; SAS Institute, Cary, NC, USA) was used for the analyses.
Results
Baseline clinical characteristics
Baseline characteristics of the included 520 patients are described in Table 1. The average age of the patients was 73 years, with a predominance of the male sex (62%), and 254 (49%) patients were older than 75 years. The average sodium excretion at entry was 3.52 ± 0.93 g/day, and that at the time of last follow-up was 3.51 ± 0.94 g/day (P = 0.75, paired t test). The average salt excretion during follow-up (from a median of 14 measurements) was 3.52 ± 0.67 g/day. Hypertension was observed in 384 (74%) patients; however, blood pressure was well controlled, with average values of 123/70 mmHg. Baseline heart disease included 220 (42%) patients with coronary artery disease, 47 (9.0%) patients with cerebrovascular disease, 97 (19%) patients with permanent atrial fibrillation (AF), and 87 (17%) patients with paroxysmal AF. We observed CHF in 114 (22%) patients, and the ejection fraction was preserved (≥ 50%) in 82 (72%) patients in this population. BNP was 53 (24–115) pg/mL (median, IQR) and was ≥ 100 pg/mL in 147 (28%) patients. The eGFR was 64 ± 18 mL/min/1.73 m2 and was < 60 mL/min/1.73 m2 in 219 (42%) patients. With regard to medications, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEI/ARBs), calcium channel blockers, and β-blockers were used in 64, 51, and 37% of cases, respectively. Diuretics were used in 125 (24%) patients, and loop diuretics were used in 92 (18%) patients. The average dose of furosemide equivalents (azosemide 30 mg = furosemide 20 mg) used in this study at the time of entry was 21 ± 10 mg (maximum 40 mg).
Endpoints
There were 105 composite CV events (3.9%/year), including 60 hospitalizations due to heart failure (2.2%/year), 9 acute coronary syndromes (0.3%/year), 21 cerebrovascular events (0.8%/year, 18 cerebral infarctions and 3 cerebral hemorrhages), and 15 CV deaths (0.6%/year), and 26 cases of all-cause mortality (1.0%/year) (Table 2). Of the 60 hospitalizations due to heart failure, 20 patients experienced new-onset ADHF, and another 40 patients had exacerbation of stable heart failure.
Table 2 shows the event rates according to sodium excretion. No differences were found between any level of sodium excretion and CV events. When we divided the level of sodium excretion into six categories, it appeared that the primary endpoint occurred more often in association with very low sodium excretion (less than 2.5 g/day), which occurred in 12 (34%) of the 35 patients in this population (Fig. 2); however, this association did not reach statistical significance as a whole (P = 0.40, chi-squared test). In addition, risk profiles (Supplementary Table 1) were higher in the patients with very low sodium excretion compared with the other 485 patients (age: 78 ± 9 vs. 73 ± 10 years [P < 0.001], body weight: 49 ± 11 vs. 61 ± 12 kg [P < 0.001], BNP: 76 (47–260) vs. 52 (23–114) pg/mL [P = 0.004], and eGFR: 57 ± 17 vs. 64 ± 18 mL/min/1.73 m2 [P = 0.042]).
Supplementary Table 1 shows the determinants of the primary endpoint in the univariate analysis. Older age, lower body weight, lower eGFR, higher BNP, lower LV ejection fraction, CHF, permanent AF, and medications (ACEI/ARBs, β-blockers, calcium channel blockers, and diuretics) were associated with the primary endpoint.
Figure 3 illustrates Kaplan–Meier primary endpoint-free curves according to estimated urinary sodium excretion. There were no significant differences in the primary endpoint among these groups (log-rank test, P = 0.70). There were also no differences in all-cause mortality among these groups (log-rank test, P = 0.10).
Kaplan–Meier primary endpoint–free curves according to estimated sodium excretion. The primary endpoint of this study is composed of (1) hospitalization due to heart failure, (2) acute coronary syndrome, (3) cerebrovascular events, and (4) CV mortality. S1 (red, < 3.0 g/day), S2 (green, 3.0–3.49 g/day), S3 (blue, 3.5–3.99 g/day), and S4 (black, ≥ 4.0 g/day)
Table 3 shows the association of estimated urinary sodium excretion with the primary endpoint and all-cause mortality. No associations were found by univariate analysis for these clinical outcomes. After adjustment for age, sex, and body weight, sodium excretion ≥ 4.0 g/day was associated with the primary endpoint (HR: 1.79, CI: 1.01–3.15), but not all-cause mortality when sodium excretion 3.0–3.49 g/day was used as a reference value. Association of sodium excretion and CV events remained consistent after further adjustments for eGFR (< 60 mL/min/1.73 m2, Model 2), but the association did not reach statistical significance after adjustment for BNP (≥ 100 pg/mL, Model 3) or use of diuretics (Model 4) (P = 0.069 and P = 0.099, respectively). On the other hand, when we used sodium excretion at the time of entry, sodium excretion was not associated with the primary or secondary endpoint (Supplementary Table 2).
Discussion
This prospective observational study demonstrated that high sodium excretion ≥ 4.0 g/day was associated with composite CV events (hospitalization due to heart failure, acute coronary syndrome, cerebrovascular events, and CV deaths), but not with all-cause mortality. The assessment was made by using repeated measurements of spot urine samples collected during the observational periods. Notably, these findings were not observed when using a single spot urine sample at the time of enrollment. The strengths of this study were that we followed up the patients for the relatively long period of 5.2 years, with few dropouts ( < 1.0% in 5 years), using a practically feasible approach in real-life clinical practice.
Two randomized control trials (RCT) have evaluated the effects of sodium intake on CV events or mortality. The first RCT, reported in Taiwan, involved the comparison of patients who were randomly assigned to receive regular salt or a combination of sodium and potassium [12]. There was a significant 35% reduction in CV mortality; however, it is not possible to know whether the outcome was due to reduced sodium or to increased potassium or both. In fact, positive associations have been reported between urinary sodium/potassium ratio and blood pressure (INTERSALT study [13]) and between urinary sodium/potassium ratio and central aortic systolic pressure in hypertensive patients in Korea [14]. A second RCT was performed in patients with heart failure in Italy [15]. There was a highly significant reduction in the combined primary endpoint of mortality and hospitalization in patients who were assigned to receive higher sodium intake of 2.8 g/day compared with lower sodium intake of 1.8 g/day during a 180-day follow-up. However, the participants were severely sodium- and water-depleted due to aggressive diuretic therapy (furosemide 250–500 mg twice daily), which is not always recommended.
Several prospective cohort studies and meta-analyses have evaluated the association between sodium intake and CV events [1, 4, 16,17,18,19,20,21,22,23], but the effects of sodium intake on CV events are controversial [24,25,26]. Some studies showed a positive association between sodium intake and CV outcomes [4, 17,18,19,20,21], two studies [22, 23] found inverse associations, and two studies found a J-shaped relationship [1, 16]. Among them, the PURE prospective cohort study [1], (n = 101,945) included a population at average CV risk, and 3.3% of the cohort experienced a major CV event or died during 3.7 years of follow-up. Compared with a sodium excretion between 4 and 5.99 g/day, both higher baseline sodium excretion (> 7 g/day) and low sodium excretion (< 3 g/day) were associated with higher risk of the composite outcomes. Another trial, the Ongoing Telmisartan Alone and in combination with Ramipril Global End point Trial (ONTARGET)/Telmisartan Randomized AssesmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) included a population at high CV risk [16], and 16.4% of the cohort experienced a major CV event or died during 5 years of follow-up. In that study, a J-shaped association between sodium intake and CV mortality was also found, with an increased risk in the groups consuming < 3 g/day of sodium and > 6 g/day. We have also shown in this study that CV events were common in the group with a high sodium intake (≥ 4.0 g/day), which might be the consequence of differences in the included population and possibly differences in race. In the subgroup of low sodium excretion (< 2.5 g/day), the primary endpoint seemed to be increased (Fig. 2). However, CV risks (older age, low body weight, high BNP, and low eGFR) were very high in these patients, and it could be due to reverse causation, although the patient number was too small (n = 35) to reach a conclusion.
The estimation of the sodium content in 24-h pooled urine is reliable and has been used in many clinical trials, but it is difficult to perform in daily outpatient medical practice because of the inconvenience of collecting urine for 24 h and inadequate urine pooling leads to underestimation of sodium intake [7, 10]. Furthermore, single 24-h urine samples are not suitable for assessing an individual’s normal sodium intake [8]. An evaluation based on dietary content determined by questionnaire or interview performed over several days requires detailed calculations by expert dieticians. Thus, this method is often not available in practical medicine and may underestimate sodium intake in certain cases because of selective statements by the patients [7, 10]. In contrast, the evaluation of sodium intake using the sodium and creatinine concentration in a spot urine sample is very easy to perform. The aforementioned two studies [1, 16] used spot urine samples only at the time of entry, in which values might not reflect sodium intake during the course of the study. To overcome this problem, we repeatedly measured sodium excretion during follow-up and used the average value (from a median of 14 measurements) for the analysis. In fact, we found no association between sodium excretion and composite CV outcomes when only sodium excretion at the time of entry was used. Thus, we believe that an assessment of sodium intake by repeated measurements of spot urine samples can be a feasible and acceptable method for a large and long-term cohort study, although this method should be validated in a future study. Assessing sodium intake using second-morning urine could be an alternative [27].
Limitations
This study has several limitations. First, selection bias is a major concern. We have tried to include every patient who visited our clinic with possible CV risks, but this in turn caused marked heterogeneity of the included patients. Further studies including patients with less severe symptoms, e.g., having only hypertension, or even from the general population might be required. Of note, our study included older patients (average 73 years at entry), so our conclusions might not always be applicable to younger patients. Second, this study was not exactly an observational study but included some interventions, i.e., dietary counseling either by attending physicians or expert dieticians. In fact, spot urine measurements had already been performed, and the results had already been notified to the majority (469, 90%) of the patients at the time of entry. Furthermore, dietary counseling by expert dieticians was frequently carried out for patients with high sodium excretion. Thus, patients included in this study did not receive a uniform degree of dietary intervention. In fact, the mean sodium excretion of 3.52 g/day in this study is much lower than that of the average Japanese population (4–4.5 g/day) [10, 11, 13], and the results of our study cannot always be applied to Japanese patients in general, or to other races. In addition, the results of sodium excretion were not concealed from the patients at each office visit. However, blinding is difficult, if not impossible, under real-life medical practice because of ethical issues. Third, our study provides an epidemiological comparison of groups that consumed different levels of sodium, and it does not provide information on the effect on clinical outcomes of reducing sodium intake. Finally, this is a single-center study with a limited number of patients. Therefore, further larger-scale, multicenter studies are needed.
Conclusions
The results of the ESPRIT study demonstrated that high sodium excretion (≥ 4.0 g/day) was associated with composite CV events (hospitalization due to heart failure, acute coronary syndrome, cerebrovascular events, and CV deaths), assessed by repeated measurements of spot urine testing. Further studies are needed to determine whether reducing sodium intake may be beneficial for the prevention of CV events.
References
O’Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, et al. PURE Investigators. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371:612–23.
Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. N Engl J Med. 2001;344:3–10.
Graudal NA, Hubeck-Graudal T, Jürgens G. Effects of low-sodium diet vs. high-sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride (Cochrane Review). Am J Hypertens. 2012;25:1–15.
Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ. 2007;334:885.
O’Donnell M, Mente A, Yusuf S. Sodium intake and cardiovascular health. Circ Res. 2015;116:1046–57.
Campbell NR, Lackland DT, Niebylski ML, Nilsson PM. Is reducing dietary sodium controversial? Is it the conduct of studies with flawed research methods that is controversial? A perspective from the world hypertension league executive committee. J Clin Hypertens. 2015;17:85–86.
Cobb LK, Anderson CA, Elliott P, Hu FB, Liu K, Neaton JD, et al. American heart association council on lifestyle and metabolic health. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the American Heart Association. Circulation. 2014;129:1173–86.
Olde Engberink RHG, van den Hoek TC, van Noordenne ND, van den Born BH, Peters-Sengers H, Vogt L. Use of a single baseline versus multiyear 24-hour urine collection for estimation of long-term sodium intake and associated cardiovascular and renal risk. Circulation. 2017;136:917–26.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Collaborators developing the Japanese equation for estimated GFR. Revis Equ Estim GFR Serum Creat Jpn Am J Kidney Dis. 2009;53:982–92.
Kawano Y, Tsuchihashi T, Matsuura H, Ando K, Fujita T, Ueshima H. Working Group for Dietary Salt Reduction of the Japanese Society of Hypertension. Report of the working group for dietary salt reduction of the japanese society of hypertension: (2) assessment of salt intake in the management of hypertension. Hypertens Res. 2007;10:887–93.
Tanaka T, Okamura T, Miura K, Kadowaki T, Ueshima H, Nakagawa H, et al. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens. 2002;16:97–103.
Chang HY, Hu YW, Yue CS, Wen YW, Yeh WT, Hsu LS, et al. Effect of potassium-enriched salt on cardiovascular mortality and medical expenses of elderly men. Am J Clin Nutr. 2006;83:1289–96.
INTERSALT Cooperative Research Group. Intersalt: an international study of electrolyte excretion and blood pressure. Result for 24 h urinary sodium and potassium excretion. Br Med J. 1988;297:319–28.
Rhee MY, Shin SJ, Gu N, Nah DY, Kim BK, Hong KS, et al. Relationship between 24-h urine sodium/potassium ratio and central aortic systolic blood pressure in hypertensive patients. Hypertens Res. 2017;40:405–10.
Paterna S, Gaspare P, Fasullo S, Sarullo FM, Di Pasquale P. Normal-sodium diet compared with low-sodium diet in compensated congestive heart failure: is sodium an old enemy or a new friend? Clin Sci (Lond). 2008;114:221–30.
O’Donnell MJ, Yusuf S, Mente A, Gao P, Mann JF, Teo K, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306:2229–38.
Tuomilehto J, Jousilahti P, Rastenyte D, Moltchanov V, Tanskanen A, Pietinen P, et al. Urinary sodium excretion and cardiovascular mortality in Finland: a prospective study. Lancet. 2001;357:848–51.
Nagata C, Takatsuka N, Shimizu N, Shimizu H. Sodium intake and risk of death from stroke in Japanese men and women. Stroke. 2004;35:1543–7.
He FJ, MacGregor GA. Salt reduction lowers cardiovascular risk: meta-analysis of outcome trials. Lancet. 2011;378:380–2.
Cook NR, Appel LJ, Whelton PK. Lower levels of sodium intake and reduced cardiovascular risk. Circulation. 2014;129:981–9.
Gardener H, Rundek T, BWright CB, Elkind MSV, Sacco RL. Dietary sodium and risk of stroke in the northern Manhattan study. Stroke. 2012;43:12001205.
Stolarz-Skrzypek K, Kuznetsova T, Thijs L, Tikhonoff V, Seidlerová J, Richart T, et al. European Project on Genes in Hypertension (EPOGH) Investigators. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA. 2011;305:1777–85.
Ekinci EI, Clarke S, Thomas MC, Moran JL, Cheong K, MacIsaac RJ, et al. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care. 2011;34:703–9.
Alderman MH, Cohen HW. Dietary sodium intake and cardiovascular mortality: controversy resolved? Am J Hypertens. 2012;25:727–34.
Alderman MH. The science upon which to base dietary sodium policy. Adv Nutr. 2014;5:764–9.
Mancia G, Oparil S, Whelton PK, McKee M, Dominiczak A, Luft FC, et al. The technical report on sodium intake and cardiovascular disease in low- and middle-income countries by the joint working group of the World Heart Federation, the European Society of Hypertension and the European Public Health Association. Eur Heart J. 2017;38:712–9.
Kawamura M, Ohmoto A, Hashimoto T, Yagami F, Owada M, Sugawara T. Second morning urine method is superior to the casual urine method for estimating daily salt intake in patients with hypertension. Hypertens Res. 2012;35:611–6.
Acknowledgements
We thank the following investigators and attending physicians, who followed the patients carefully and gave us important information, for their participation in this study. Masatoshi Fuyuta, Takafumi Hirata, Kenji Horiuchi, Shinsuke Hanatani, Masayuki Imafuji, Yoichi Itoyama, Hidemasa Kobayashi, Tetsuro Kuwahara, Mitsuhiro Matsumoto, Yoshiharu Murohara, Hisaki Narita, Keisuke Ohba, Yasuo Okubo, Eiji Otsuka, Haruki Shimizu, and Hidefumi Yasunari. We thank Mrs. Akiko Hatano and Mrs. Tomomi Fukuoka for their secretarial assistance. We especially thank Dr. Takuya Tsuchihashi, Steel Memorial Yawata Hospital, and Dr. Yusuke Ohya, Ryukyu University, for their critical review of this paper and helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Sadanaga, T., Hirota, S., Enomoto, K. et al. Evaluation of sodium intake for the prediction of cardiovascular events in Japanese high-risk patients: the ESPRIT Study. Hypertens Res 42, 233–240 (2019). https://doi.org/10.1038/s41440-018-0149-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0149-7
Keywords
This article is cited by
-
Meta-analytic research of the dose-response relationship between salt intake and risk of heart failure
Hypertension Research (2021)
-
Sodium status is associated with type 2 diabetes mellitus: a systematic review and meta-analysis of observational studies
European Journal of Nutrition (2021)
-
Factors associated with heart failure hospitalization in patients with high sodium excretion: subanalysis of the ESPRIT, evaluation of sodium intake for the prediction of cardiovascular events in Japanese high-risk patients, cohort study
Heart and Vessels (2021)
-
The optimal amount of salt intake
Hypertension Research (2019)