Abstract
Long-term blood pressure variations contribute to an increased risk of cardiovascular events during cold season, requiring personalized management of antihypertensive medications in a single patient, and can influence the results of clinical trials and epidemiological surveys in population studies. In addition to blood pressure values, which guide the stratification of cardiovascular risk, other cardiovascular risk factor levels also tend to be higher in the winter months and lower in the summer months. The resultant estimation of individual cardiovascular risk may thus vary depending on the season. At the patient level, only a low value in the winter should thus be considered a true measure of low cardiovascular risk, whereas low values measured in the summer do not indicate a low risk in the winter. Likewise, estimations of cardiovascular risk in population studies may vary according to the period of the year. Efforts should thus be directed at considering the potential influence of seasonal variations in establishing “normal” and “high-risk” assessment at both the patient and population levels, integrating such data into clinical practice.
Similar content being viewed by others
Introduction
Both physicians and patients are aware of seasonal fluctuations in blood pressure (BP). In clinical practice, it is not uncommon to find patients with mild hypertension requiring pharmacological treatment only in the winter, whereas in the summer, they can suspend or reduce drug intake. However, despite documented observations on the clinical influence of seasonality, the importance of environmental variations is often neglected.
Although the influence of temperature on BP has been known since the 1920s, the lowering of BP in the summer was first described in 1961 by Rose [1] in a small cohort of 56 men observed for 1–3 years at a clinic for ischemic heart disease. In the UK Heart Disease Prevention Project [2], an inverse relationship between diastolic pressure and the temperature of the room in which BP measurements were made was observed. In the same study, standardization of room temperature largely removed the seasonal effect [2]; thus, the importance of standardized room temperature in hypertension clinics became evident. The importance of considering seasonality in controlled clinical trials became evident with the Medical Research Council (MRC) treatment trial [3], where over 17,000 men and women (aged 35 to 64 years) with diastolic blood pressures (DBP) of 90 to 109 mm Hg were recruited, randomized to placebo, bendrofluazide, and propranolol. During the 5-year follow-up, seasonality was found to be associated with larger BP variations than those induced by drugs, although most of the measurements were made in comfortably warm rooms [3], changes being more evident in older people. More precisely, in men aged 55–64 years, office systolic BP (SBP) was 6–7mm Hg higher and DBP was 3–4mm Hg higher on winter days than on summer days; in men aged 35–44 years, the comparable figures were increases of 2–4 mm Hg SBP and 2–3 mm Hg DBP. Similar changes were observed in women, with greater changes in thinner people than in more obese ones [3]. These changes were only attenuated by compliance with guidelines that constantly recommended the importance of a standardized room temperature in hypertension clinics because even when the standards for BP measurement were met, seasonal variations continued to be evident. A similar range of seasonal SBP variation (between 5 mm Hg and 7 mm Hg) was seen in the temperate climates of Japan, Great Britain, Minnesota, Austria, Italy, and Pennsylvania [4,5,6]. Seasonal BP disparities were also studied in subtropical areas. In India, normotensive women aged 18 to 40 and living in Delhi had BPs values 11.07⁄6.79 mm Hg higher in the winter than in the summer [7]. Hypertensive Israeli patients aged 65 to 91 years had on average BPs of 165⁄90 mm Hg in the winter compared with 134⁄74 mm Hg in the summer [8]. In Mosul, Iraq, the mean summer BP for hypertensive patients was 130.6⁄85 mm Hg compared with 147.6⁄94.1 mm Hg for the same patients during the winter [9]. In the 2051 subjects enrolled in the Pressione Arteriose Monitorate E Loro Associazioni (PAMELA) study, seasonal BP changes were also observed for home and ambulatory BP (ABP) (average 24 h) [10].
In addition, a reduced intensity in ultraviolet light during the winter might also reduce epidermal photosynthesis of vitamin D3 and parathyroid hormone, which was shown in turn to be associated with elevated BP levels [11]. Seasonal variation in many behavioral factors, such as diet [12,13,14,15] or physical activity (amplitude, type, and intensity of activity increasing in summer), were also observed [16,17,18]. The possible influence of sodium and potassium consumption on seasonal BP patterns has been recently investigated [19]. The maximum impact of seasonal variation in sodium consumption on SBP ranged from 0.4 to 1.1 mm Hg, depending on the model considered. These variations do not explain the seasonal variations in BP levels [19]. The independent role of temperature in seasonal variations must therefore be carefully considered.
Seasonal BP variations: does temperature have an independent role?
Much of the discussion on the winter season increase in BP has been centered on the role of temperature. As the temperature falls, blood vessels constrict, which leads to increased resistance in the peripheral circulation and an increase in blood pressure. Cold pressor responses are age related; younger patients were reported to exhibit a greater increase in peripheral vascular resistance, whereas older patients exhibited a greater increase in cardiac output [4]. In the French Three-City study, where the relationship between office BP measured according to current guidelines and outdoor temperatures obtained from local meteorological office was prospectively investigated in 8801 participants over the age of 65 years, BP decreased by 8.0 mm Hg between the lowest ( < 7.9 °C) and the highest (21.2 °C) temperatures [20]. These relationships were independent of anthropometric data and baseline BP values and were rather related to subject age. BP changes were greater in subjects 80 years or older than in younger participants. A negative relationship between outdoor temperature and BP values was consistently observed [21].
The possible influence of temperature in estimating hypertension prevalence in an epidemiological survey was specifically assessed in a large population study performed in Yemen on 12,000 people aged 15 to 69 years [22]. The varied Yemen topography corresponds to widespread climate changes, with a temperate climate in the highlands and high temperatures in the coastal region. In the HYDY study, the air temperature measured indoors during BP measurement was negatively associated with hypertension prevalence, and each 1 °C temperature increase was associated with a 0.2% significant reduction of hypertension prevalence with an odds ratio of 0.98 (95% confidence interval (Cl) 0.96 to 0.99) in logistic regression analyses adjusted for age, gender, education, and average air temperature at the two survey visits [22]. Importantly, the geographic distribution of hypertension burden follows an opposite trend than the average air temperature measured during home visits, as hypertension prevalence was higher at the coast (with an average temperature of 28.3 °C; 95% CI 28.1 to 28.4 °C) than in the highlands (average temperature of 22.0 °C; 95% CI 21.9 to 22.1 °C). Air temperature may thus only modulate the contribution of other factors, with a limited but significant final effect. The inclusion of indoor temperature among data collected in epidemiological surveys should thus be considered. The importance of room temperature on the variation of home blood pressure was shown in a recent study that analyzed 87,535 morning and evening BP readings acquired through a long-term cohort study performed in 106 subjects living in a rural community in northern Japan [23]. More precisely, outside temperature showed a greater summer–winter difference than room temperature; however, room temperature showed a larger variation within a month compared with outside temperature, which was likely due in part to differences in the usage of heating and air conditioning among subjects [23]. These studies stress the importance of “Internet of Things” technology with network-embedded blood pressure monitors and thermostats for better future management of hypertension [23].
Associations of SBP with seasons and indoor and outdoor temperatures and interactions with sex and short-term temperature trends and body mass index (BMI) were investigated in 25 populations in 16 countries from the World Health Organization MONICA (monitoring trends and determinants in cardiovascular disease) project risk factor surveys covering the years 1979–1997 in which the measurements were reasonably spread throughout the year [24]. Season had a significant effect on SBP, with a mean BP increase of 2.06 mm Hg (95% posterior interval (PI) 1.05 to 3.08 mm Hg) midwinter (compared with midsummer). However, the effects of the winter season disappeared after controlling for outdoor temperature, suggesting that a major component of the seasonal change in BP was the direct result of temperature. The mean effect of outdoor temperature was smaller than that of indoor temperature because for each 1 °C increase in outdoor and indoor temperature, SBP fell by 0.31 mm Hg (95% PI: –0.44 to –0.19 mm Hg) and 0.19 mm Hg (95% PI: –0.26 to –0.11 mm Hg), respectively. However, the effect of outdoor temperature remained after controlling for indoor temperature, as a 1 °C increase in outdoor temperature reduced BP values by 0.14 mm Hg (95% Cl −0.23 to −0.05, adjusted for indoor temperature). In most populations, increases in short-term temperature trends were associated with lower blood pressure, and in 3 of the 18 populations, this association was statistically significant [24].
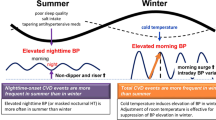
A different line of evidence seems to indicate that an effect of seasonality independent of temperature may exist. In the PAMELA study, seasonal changes in BP were less pronounced when average 24-h ambulatory pressures were compared to office measurements [4]. Records of daily home BP measurements also enable the capture of long-term factors, such as seasonal variation [25]. During the summer, the reduction in daytime BP values is indeed associated with a significant increase in nighttime BP values, resulting in an increased prevalence of non-dippers in the summer [26,27,28,29]. In conclusion, all aspects of the BP profile, except for nighttime ABP, are reduced in the summer, resulting in an increased prevalence of non-dippers in the summer with unknown consequences [28]. Conversely, the acute onset of a cold weather front is followed within 2 days by a concordant increase of 24 h, daytime, and nighttime ambulatory BP [30]. These observations suggest that an independent effect of seasonality can be evident on nighttime BP.
A recent study [31] was specifically performed to disentangle the effects of temperature on BP from those of seasonality. The number of hours between sunrise and sunset was indeed included in multiple regression analysis as a continuous measure of seasonality. The study for the first time provided evidence that daytime SBP was independently affected by air temperature, whereas nighttime SBP and morning BP surge were mainly affected by seasonality [31]. The direct effect of temperature on 24 h SBP was evident in subjects aged more than 65 years, thus indicating that temperature-associated 24 h ambulatory BP change is more pronounced with aging (Table 1) [31]. Nighttime BP thus seems to be mainly related with seasonality, with temperature mainly affecting daytime BP values.
Therefore, BP assessment in a single patient might yield different results when performed in hot months (summer) or cold months (winter). Likewise, estimations in population studies may vary according to the time of year [21]. On the other hand, a non-random distribution of enrollments over the year can bias the results of clinical trials aimed at assessing the antihypertensive effect of a drug and the findings of epidemiological surveys aimed at assessing hypertension burden.
Implications for cardiovascular risk stratification
BP assessment in a single patient might thus give different results when performed in hot months (summer) or cold months (winter). The influence of seasonality and other environmental factors on arterial stiffness and wave reflection has been recently analyzed [32]. Daily number of hours of light was found to independently affect the measurements of carotid–femoral pulse wave velocity in hypertensive patients, with the association being stronger in women and in untreated patients than in men or treated patients, respectively [32].
According to cross-sectional data from 24 population-based studies from 15 countries with a total sample size of 237,979 subjects, seasonal BP changes are also associated with changes in other cardiovascular (CV) risk factor levels (plasma high-density lipoprotein (HDL), low-density lipoprotein (LDL), glucose, and BMI), which also tended to be higher in the winter months and lower in the summer months [33]. In the Northern Hemisphere, estimated seasonal variations were 0.26 kg/m2 for BMI, 0.6 cm for waist circumference, 2.9 mm Hg for SBP, 1.4 mm Hg for DBP, 0.02 mmol/L for triglycerides, 0.10 mmol/L for total cholesterol, 0.01 mmol/L for HDL cholesterol, 0.11 mmol/L for LDL cholesterol, and 0.07 mmol/L for glycemia, which are similar seasonal variations found in the Southern Hemisphere [33]. Statistically significant seasonal rhythms were detected for plasma glucose, triacylglycerides, free fatty acids, free cholesterol and esterified cholesterol, cholesterol in LDLs, cholesterol in HDLs, phospholipids, and lipase activities in a cohort of 245 healthy students between 18 and 25 years old, with most of these values peaking in the winter season [34].
The seasonality and effects of temperature on levels of fasting plasma glucose were also investigated in China among 49,417 participants who had three health examinations [35], and fasting plasma glucose levels were found to be higher in the winter and spring than in the autumn and summer [35]. Seasonal variation of hemoglobin A1c (HbA1c) was investigated in a hospital-based adult population over a period of 5 years and included 62,384 HbA1c valid measurements [36]. The peak level was found in January–February (7.1%), a trough was observed in August–October (6.8%), and an average peak-to-trough amplitude value of 0.3% was determined. This trend was observed in in the evaluated genders and age subgroups [36]. Therefore, seasonality in the population average blood glucose levels presumably reflects a general periodic tendency in individuals for winter glucose levels to be higher than summer levels [37]. A different pattern with a peak in the summer was reported for the diagnosis of gestational diabetes mellitus [38, 39].
Seasonal variation in the prevalence rates of metabolic syndrome was also recently prospectively studied in a cohort of 758 Japanese male workers (40–65 years of age) who underwent the homeostasis model assessment of insulin resistance in the summer and winter of 2010 [40]. Metabolic syndrome, defined according to the criteria proposed by the International Diabetes Federation and the Japanese Society of Internal Medicine, significantly increased from 12.4 and 9.6% in the summer to 16.6 and 13.3% in the winter [40].
These findings indicate that besides blood pressure, plasma HDL, LDL, and glucose are also slightly higher in the winter than in the summer, and it has been reported that more patients on statin therapy achieve their target LDL level in the summer than in the winter, suggesting that plasma lipoprotein metabolism in humans may be regulated by the seasons [41]. Attention towards the environmental determinants of cardiovascular disease is growing [42] because the resultant estimation of individual CV risk may vary depending on the season. More precisely, at the patient level, only a low value in the winter should thus be considered a true low risk, whereas low values measured in summer do not indicate a low risk in the winter.
In common clinical practice, it is not usual to observe patients requiring seasonal changes in antihypertensive drug treatment. Seasonal adaptation of antihypertensive drugs is not specifically considered in hypertension guidelines because treatment targets are defined by BP values. However, this absence probably also reflects a lack of interventional studies that provide evidence as to when and how intervention in these cases is necessary and beneficial. However, in daily clinical practice, physicians often face with the effects of warm temperature, which may cause a reduction in BP during the summer with the potential implication of falls or acute renal failure, especially in the elderly. Coupled with reduced fluid intake, there is a decrease in total body water with advancing age. The independent association between BP increase at nighttime and seasonality in the elderly [31] might remain against this simplistic position. Likewise, the daily number of hours of light and age independently affect the measurements of PWV in hypertensive patients, with stronger associations in women and untreated patients than in their counterparts [32]. Because of their low water reserves, the elderly are encouraged to learn to drink regularly when they are not thirsty and to moderately increase their salt intake when they sweat [43]. “Pusher” advertisements from the media suggest that drinking water to avoid more urine exerts favorable effects on the kidney can be received more favorably by older patients, even with those with heart failure, than the risk advice provided by physicians [44]. The large majority of experimental studies are confined to short-term (up to few days) exposition of aged subjects to high temperature, whereas no information is available on blood volume adaptation in the long term. It might thus be hypothesized that blood volume adaptation, resulting in increased BP at nighttime, might occur in the long term. This response might be modulated between the spring and summer because night BP levels are the highest in the spring; however, the daily hours of light is at its highest level in the summer.
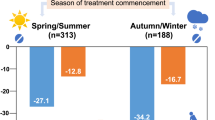
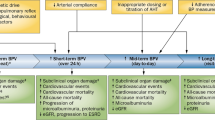
At the population level, CV risk estimation plays a key role in the efficient allocation of health resources [45] and in planning improvements in the quality of housing [46]. The increase in mortality was reported to be 1% per 1 °C fall in temperature [47]. Daily event rates of myocardial infarction were observed to be significantly increased with daily mean air temperature decrease, and a 10 °C decrease was associated with a 19% increase in daily event rates for people older than 65 years [48, 49]. According to a recent meta-analysis, the incidence of aortic dissection is significantly more frequent in the winter (28.2%) than in other seasons, and the incidence is significantly less frequent in the summer (20.6%) than in other seasons (winter>spring ≈ autumn>summer) [50]. Likewise, the seasons influence the occurrence of aneurysmal subarachnoid hemorrhage, with aneurysmal subarachnoid hemorrhage occurring less often in the summer than in the winter and most often in January [51]. Cross-sectional and observation surveys indicate that health interventions targeted at better protection against cold weather (e.g., improved home heating and reduced exposition to cold climate) may be particularly effective in the elderly [43, 48, 52]. In the reanalysis of BP data collected within the WHO MONICA Project, the random effects that seasons had on BP were latitude dependent (regression slope: –0.17, 95% confidence interval: –0.27 to –0.09), being lower for countries with colder climates and higher for countries with warmer climates [24]. Conversely, no association between the estimated amplitude of seasonal BP variations and latitude was observed by Marti-Soler et al. [33]. The number of populations and countries included in the two studies are comparable; the model adopted to estimate seasonal BP variations is also similar. Nevertheless, a large majority of studies included by Marti-Soler et al. [33] were performed after 1997 (only 8 out of the 23 studies were started before 1997), whereas the collection period of the studies included by Barnett et al. [24] ranged from 1979 to 1997. Implications for low-income countries can hardly be drawn because of the limited number of studies carried out in the Southern Hemisphere and because of the absence of studies investigating populations from Asia, Africa, and South America. When focusing our attention on Europe, it is to be considered that a temperature series in the United Kingdom has exhibited an increase of approximately 0.8 °C over the past two or three decades. In England and Wales, the association of year-to-year variation in excess winter mortality with the number of cold days in winter (<5°C), which was evident until mid-1970, has recently disappeared, and the link between winter and excess mortality is no longer as strong as before [53, 54]. In a large study of over 23,000 individuals with prior CVD who were recruited from general communities in 10 diverse regions of China, substantial seasonal variation in BP was observed, especially in areas where there was little use of central heating in the cold months [55]. Climate warming might bring benefits, at least to disadvantaged populations.
Seasonal BP variations and visit-to-visit BP variability
In addition to seasonal BP changes, visit-to-visit SBP variability was also reported to be associated with stroke and CV mortality [56]. A differential influence of different antihypertensive BP drugs on visit-to-visit SBP variability was reported [57,58,59,60,61], although their influence on stroke was not observed. More precisely, retrospective analyses of published trial data have concluded that antihypertensive drug classes may differ in their effects on intersession visit-to-visit BP variability and associated risk of stroke [57,58,59,60,61,62]. In a retrospective study, single-pill fixed-dose combinations of Angiotensin II receptor blocker (ARB)/Calcium channel blocker (CCB) and ARB/diuretic (DI) were reported to have similar effects on visit-to-visit variability and seasonal variation in BP in hypertensive patients [63]. However, these post hoc analyses lacked actual intersession information for individual trial participants, adherence to drugs, duration of drug action, or adjustment for temperature or seasonality.
Low adherence to antihypertensive medication has been hypothesized to increase the visit-to-visit variability of BP.
The association between antihypertensive medication adherence and visit-to-visit variability of BP was recently studied in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) [64]. The visit-to-visit variability of SBP and DBP was higher among non-adherent participants than among adherent participants. However, adjustment for nonadherence did not explain the association of visit-to-visit variability of BP with higher fatal coronary heart disease or nonfatal myocardial infarction, stroke, heart failure, or mortality risk [64].
The relationship between BP variability and seasonal effects on the renal outcome was investigated in a recent study in which a combination therapy with ARB and a CCB vs ARB plus diuretics were compared in terms of decreasing BP variability in hypertensive patients [60]. Multivariate regression analyses using outdoor temperature and daylight hours as independent variables revealed no significant correlations between daylight hours and urine albumin-to-creatinine ratio (UACR) or estimated glomerular filtration rate (eGFR) as dependent variables [60]. However, there was a correlation between outdoor temperature and both UACR and eGFR in the ARB+D group. This finding suggests that seasonal effects on the renal outcome may exist, especially with diuretic combination therapy [60].
The degree of risk of stroke based on visit-to-visit SBP variability was recently investigated in a large Chinese hypertensive population in 32 communities [65]. The hazard ratios for risk of stroke increased by quintiles of visit-to-visit SBP standard deviation, coefficient of variation, and average real variability over 6 visits (1st quintile, Q1, reference). Advanced age and BMI ≥25 kg/m2 were associated with increased risk. However, temperature was not included among the investigated variables [65]. Therefore, the interaction between seasonal BP changes and visit-to-visit SBP variability on CV events remains to be assessed.
Conclusions
Seasonal office BP changes are mainly influenced by temperature. The control of concurrent environmental changes should be considered in both clinical practice and research studies. In daily clinical practice, ABP is important in monitoring antihypertensive treatment in elderly patients under conditions of unstable and often “extreme” temperature exposure. Short-term temperature changes mainly affect BP during the daytime (temperature as a negative predictor), whereas seasonality mainly affects nighttime SBP (with daylight hours as a positive predictor) and morning BP surge (with daylight hours as a negative predictor). The design of clinical trials should consider the months of enrollments, with long-term seasonal BP variations being especially relevant in the elderly. Population surveys in general should routinely factor in seasonal variations in blood pressure. Epidemiological surveys aimed at estimating hypertension burden in a community may include indoor measurements of environmental temperature as a variable potentially affecting results rather than considering outdoor temperature only. Seasonal BP changes and visit-to-visit SBP variability were both reported to be associated with stroke and CV mortality. However, the interaction between seasonal BP changes and visit-to-visit SBP variability remains to be assessed. Awareness of these phenomena will result in more personalized, tailored dosages of antihypertensive medications.
References
Rose G. Seasonal variation in blood pressure in man. Nature. 1961;189:235.
Heller RF, Rose G, Pedoe HD, Christie DG. Blood pressure measurement in the united kingdom heart disease prevention project. J Epidemiol Community Health. 1978;32:235–8.
Brennan PJ, Greenberg G, Miall WE, Thompson SG. Seasonal variation in arterial blood pressure. Br Med J (Clin Res Ed). 1982;285:919–23.
Rosenthal T. Seasonal variations in blood pressure. Am J Geriatr Cardiol. 2004;13:267–72.
Giaconi S, Ghione S, Palombo C, Genovesi-Ebert A, Marabotti C, Fommei E, Donato L. Seasonal influences on blood pressure in high normal to mild hypertensive range. Hypertension. 1989;14:22–27.
Thomas C, Wood GC, Langer RD, Stewart WF. Elevated blood pressure in primary care varies in relation to circadian and seasonal changes. J Hum Hypertens. 2008;22:755–60.
Sinha P, Kumar TD, Singh NP, Saha R. Seasonal variation of blood pressure in normotensive females aged 18 to 40 years in an urban slum of Delhi, India. Asia Pac J Public Health. 2010;22:134–45.
Charach G, Rabinovich PD, Weintraub M. Seasonal changes in blood pressure and frequency of related complications in elderly Israeli patients with essential hypertension. Gerontology. 2004;50:315–21.
Al-Tamer YY, Al-Hayali JM, Al-Ramadhan EA. Seasonality of hypertension. J Clin Hypertens (Greenwich). 2008;10:125–9.
Sega R, Cesana G, Bombelli M, Grassi G, Stella ML, Zanchetti A, Mancia G. Seasonal variations in home and ambulatory blood pressure in the PAMELA population. Pressione Arteriose Monitorate e Loro Associazioni. J Hypertens. 1998;16:1585–92.
Rostand SG. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30:150–6.
Hopstock LA, Barnett AG, Bonaa KH, Mannsverk J, Njolstad I, Wilsgaard T. Seasonal variation in cardiovascular disease risk factors in a subarctic population: the Tromso Study 1979-2008. J Epidemiol Community Health. 2013;67:113–8.
Shahar DR, Froom P, Harari G, Yerushalmi N, Lubin F, Kristal-Boneh E. Changes in dietary intake account for seasonal changes in cardiovascular disease risk factors. Eur J Clin Nutr. 1999;53:395–400.
Hadaegh F, Harati H, Zabetian A, Azizi F. Seasonal variability of serum lipids in adults: Tehran lipid and glucose study. Med J Malays. 2006;61:332–8.
Zhou X, Lin H, Zhang S, Ren J, Wang Z, Zhang Y, Wang M, Zhang Q. Effects of climatic factors on plasma lipid levels: a 5-year longitudinal study in a large Chinese population. J Clin Lipidol. 2016;10:1119–28.
Matthews CE, Freedson PS, Hebert JR, Stanek EJ, Merriam PA, Rosal MC, Ebbeling CB, Ockene IS. Seasonal variation in household, occupational, and leisure time physical activity: longitudinal analyses from the seasonal variation of blood cholesterol study. Am J Epidemiol. 2001;153:172–83.
Wuerzner G, Bochud M, Zweiacker C, Tremblay S, Pruijm M, Burnier M. Step count is associated with lower nighttime systolic blood pressure and increased dipping. Am J Hypertens. 2013;26:527–34.
O’Connell SE, Griffiths PL, Clemes SA. Seasonal variation in physical activity, sedentary behaviour and sleep in a sample of UK adults. Ann Hum Biol. 2014;41:1–8.
Marti-Soler H, Pommier C, Bochud M, Guessous I, Ponte B, Pruijm M, Ackermann D, Forni Ogna V, Paccaud F, Burnier M, Pechere-Bertschi A, Devuyst O, Marques-Vidal P. Seasonality of sodium and potassium consumption in Switzerland. Data from three cross-sectional, population-based studies. Nutr Metab Cardiovasc Dis. 2017;27:792–8.
Alperovitch A, Lacombe JM, Hanon O, Dartigues JF, Ritchie K, Ducimetiere P, Tzourio C. Relationship between blood pressure and outdoor temperature in a large sample of elderly individuals the three-city study. Arch Intern Med. 2009;169:75–80.
Modesti PA. Season, temperature and blood pressure: a complex interaction. Eur J Intern Med. 2013;24:604–7.
Modesti PA, Bamoshmoosh M, Rapi S, Massetti L, Al-Hidabi D, Al Goshae H. Epidemiology of hypertension in Yemen: effects of urbanization and geographical area. Hypertens Res. 2013;36:711–7.
Yatabe J, Yatabe MS, Morimoto S, Watanabe T, Ichihara A. Effects of room temperature on home blood pressure variations: findings from a long-term observational study in Aizumisato town. Hypertens Res. 2017;40:785–7.
Barnett AG, Sans S, Salomaa V, Kuulasmaa K, Dobson AJ. The effect of temperature on systolic blood pressure. Blood Press Monit. 2007;12:195–203.
Hanazawa T, Asayama K, Watabe D, Hosaka M, Satoh M, Yasui D, Obara T, Inoue R, Metoki H, Kikuya M, Imai Y, Ohkubo T. Seasonal variation in self-measured home blood pressure among patients on antihypertensive medications: HOMED-BP study. Hypertens Res. 2017;40:284–90.
Modesti PA, Morabito M, Bertolozzi I, Massetti L, Panci G, Lumachi C, Giglio A, Bilo G, Caldara G, Lonati L, Orlandini S, Maracchi G, Mancia G, Gensini GF, Parati G. Weather-related changes in 24-hour blood pressure profile: effects of age and implications for hypertension management. Hypertension. 2006;47:155–61.
Fedecostante M, Barbatelli P, Guerra F, Espinosa E, Dessi-Fulgheri P, Sarzani R. Summer does not always mean lower: seasonality of 24 h, daytime, and night-time blood pressure. J Hypertens. 2012;30:1392–8.
Stergiou GS, Myrsilidi A, Kollias A, Destounis A, Roussias L, Kalogeropoulos P. Seasonal variation in meteorological parameters and office, ambulatory and home blood pressure: predicting factors and clinical implications. Hypertens Res. 2015;38:869–75.
Wong LH, Ting P, Kerins D. Seasonal variations in nocturnal changes in blood pressure between Ireland and Singapore. Clin Trials Regul Sci Cardiol. 2015;12:12–17.
Morabito M, Crisci A, Orlandini S, Maracchi G, Gensini GF, Modesti PA. A synoptic approach to weather conditions discloses a relationship with ambulatory blood pressure in hypertensives. Am J Hypertens. 2008;21:748–52.
Modesti PA, Morabito M, Massetti L, Rapi S, Orlandini S, Mancia G, Gensini GF, Parati G. Seasonal blood pressure changes: an independent relationship with temperature and daylight hours. Hypertension. 2013;61:908–14.
Di Pilla M, Bruno RM, Stea F, Massetti L, Taddei S, Ghiadoni L, Modesti PA. Impact of seasonality and air pollutants on carotid-femoral pulse wave velocity and wave reflection in hypertensive patients. PLoS ONE. 2017;12:e0172550.
Marti-Soler H, Gubelmann C, Aeschbacher S, Alves L, Bobak M, Bongard V, Clays E, de Gaetano G, Di Castelnuovo A, Elosua R, Ferrieres J, Guessous I, Igland J, Jorgensen T, Nikitin Y, O’Doherty MG, Palmieri L, Ramos R, Simons J, Sulo G, Vanuzzo D, Vila J, Barros H, Borglykke A, Conen D, De Bacquer D, Donfrancesco C, Gaspoz JM, Giampaoli S, Giles GG, Iacoviello L, Kee F, Kubinova R, Malyutina S, Marrugat J, Prescott E, Ruidavets JB, Scragg R, Simons LA, Tamosiunas A, Tell GS, Vollenweider P, Marques-Vidal P. Seasonality of cardiovascular risk factors: an analysis including over 230 000 participants in 15 countries. Heart. 2014;100:1517–23.
Cambras T, Baena-Fustegueras JA, Pardina E, Ricart-Jane D, Rossell J, Diez-Noguera A, Peinado-Onsurbe J. Seasonal variation in plasma lipids and lipases in young healthy humans. Chronobiol Int. 2017;34:1248–58.
Li S, Zhou Y, Williams G, Jaakkola JJ, Ou C, Chen S, Yao T, Qin T, Wu S, Guo Y. Seasonality and temperature effects on fasting plasma glucose: a population-based longitudinal study in china. Diabetes Metab. 2016;42:267–75.
Pereira MT, Lira D, Bacelar C, Oliveira JC, de Carvalho AC. Seasonal variation of haemoglobin A1c in a Portuguese adult population. Arch Endocrinol Metab. 2015;59:231–5.
Kershenbaum A, Kershenbaum A, Tarabeia J, Stein N, Lavi I, Rennert G. Unraveling seasonality in population averages: an examination of seasonal variation in glucose levels in diabetes patients using a large population-based data set. Chronobiol Int. 2011;28:352–60.
Katsarou A, Claesson R, Ignell C. Seasonal pattern in the diagnosis of gestational diabetes mellitus in Southern Sweden. J Diabetes Res. 2016;2016:8905474
Chiefari E, Pastore I, Puccio L, Caroleo P, Oliverio R, Vero A, Foti DP, Vero R, Brunetti A. Impact of seasonality on gestational diabetes mellitus. Chronobiol Int. 2017;17:246–52.
Kamezaki F, Sonoda S, Nakata S, Muraoka Y, Okazaki M, Tamura M, Abe H, Tekeuchi M, Otsuji Y. Association of seasonal variation in the prevalence of metabolic syndrome with insulin resistance. Hypertens Res. 2013;36:398–402.
Tung P, Wiviott SD, Cannon CP, Murphy SA, McCabe CH, Gibson CM. Seasonal variation in lipids in patients following acute coronary syndrome on fixed doses of Pravastatin (40 mg) or Atorvastatin (80 mg) (from the Pravastatin or Atorvastatin Evaluation and Infection Therapy-thrombolysis in Myocardial Infarction 22 [PROVE IT-TIMI 22] study). Am J Cardiol. 2009;103:1056–60.
Bhatnagar A. Environmental determinants of cardiovascular disease. Circ Res. 2017;121:162–80.
Brook RD, Weder AB, Rajagopalan S. “Environmental hypertensionology” the effects of environmental factors on blood pressure in clinical practice and research. J Clin Hypertens (Greenwich). 2011;13:836–42.
Bartoli E, Rossi L, Sola D, Castello L, Sainaghi PP, Smirne C. Use, misuse and abuse of diuretics. Eur J Intern Med. 2017;39:9–17.
Modesti PA, Rapi S, Bamoshmoosh M, Baldereschi M, Massetti L, Padeletti L, Gensini GF, Zhao D, Al-Hidabi D, Al Goshae H. Impact of one or two visits strategy on hypertension burden estimation in HYDY, a population-based cross-sectional study: implications for healthcare resource allocation decision making. BMJ Open. 2012;2:e001062.
Baker-Blocker A. Winter weather and cardiovascular mortality in Minneapolis-St. Paul. Am J Public Health. 1982;72:261–5.
Nayha S. Cold and the risk of cardiovascular diseases. A review. Int J Circumpolar Health. 2002;61:373–80.
Morabito M, Modesti PA, Cecchi L, Crisci A, Orlandini S, Maracchi G, Gensini GF. Relationships between weather and myocardial infarction: a biometeorological approach. Int J Cardiol. 2005;105:288–93.
Danet S, Richard F, Montaye M, Beauchant S, Lemaire B, Graux C, Cottel D, Marecaux N, Amouyel P. Unhealthy effects of atmospheric temperature and pressure on the occurrence of myocardial infarction and coronary deaths. A 10-year survey: The Lille-world Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease). Circulation. 1999;100:E1–7.
Takagi H, Ando T, Umemoto T. Meta-analysis of seasonal incidence of aortic dissection. Am J Cardiol. 2017;120:700–7.
de Steenhuijsen Piters WA, Algra A, van den Broek MF, Dorhout Mees SM, Rinkel GJ. Seasonal and meteorological determinants of aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Neurol. 2013;260:614–9.
Morabito M, Crisci A, Vallorani R, Modesti PA, Gensini GF, Orlandini S. Innovative approaches helpful to enhance knowledge on weather-related stroke events over a wide geographical area and a large population. Stroke. 2011;42:593–600.
Kinney PL, Schwartz J, Pascal M, Petkova E, Tertre AL, Medina S, Vautard R. Winter season mortality: will climate warming bring benefits? Environ Res Lett. 2015;10:064016.
Modesti PA. The hazard of rounding cape horn: Is it changing? Heart. 2014;100:1489–90.
Yang L, Li L, Lewington S, Guo Y, Sherliker P, Bian Z, Collins R, Peto R, Liu Y, Yang R, Zhang Y, Li G, Liu S, Chen Z, China Kadoorie Biobank Study Collaboration. Outdoor temperature, blood pressure, and cardiovascular disease mortality among 23 000 individuals with diagnosed cardiovascular diseases from China. Eur Heart J. 2015;36:1178–85.
Wang J, Shi X, Ma C, Zheng H, Xiao J, Bian H, Ma Z, Gong L. Visit-to-visit blood pressure variability is a risk factor for all-cause mortality and cardiovascular disease: a systematic review and meta-analysis. J Hypertens. 2017;35:10–17.
Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlof B, Poulter NR, Sever PS, ASCOT-BPLA and MRC Trial Investigators Investigators MRCT. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469–80.
Muntner P, Levitan EB, Lynch AI, Simpson LM, Whittle J, Davis BR, Kostis JB, Whelton PK, Oparil S. Effect of chlorthalidone, amlodipine, and lisinopril on visit-to-visit variability of blood pressure: results from the antihypertensive and lipid-lowering treatment to prevent heart attack trial. J Clin Hypertens (Greenwich). 2014;16:323–30.
Rakugi H, Ogihara T, Saruta T, Kawai T, Saito I, Teramukai S, Shimada K, Katayama S, Higaki J, Odawara M, Tanahashi N, Kimura G. Preferable effects of olmesartan/calcium channel blocker to olmesartan/diuretic on blood pressure variability in very elderly hypertension: Colm study subanalysis. J Hypertens. 2015;33:2165–72.
Sato N, Saijo Y, Sasagawa Y, Morimoto H, Takeuchi T, Sano H, Koyama S, Takehara N, Morita K, Sumitomo K, Maruyama J, Kikuchi K, Hasebe N. Visit-to-visit variability and seasonal variation in blood pressure: combination of antihypertensive therapy in the elderly, multicenter investigation (CAMUI) trial subanalysis. Clin Exp Hypertens. 2015;37:411–9.
Umemoto S, Ogihara T, Matsuzaki M, Rakugi H, Ohashi Y, Saruta T. Effects of calcium channel blocker-based combinations on intra-individual blood pressure variability: post hoc analysis of the COPE trial. Hypertens Res. 2016;39:46–53.
Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet. 2010;375:906–15.
Shiga Y, Miura S, Adachi S, Suematsu Y, Sugihara M, Iwata A, Yahiro E, Nishikawa H, Ogawa M, Saku K. Visit-to-visit variability and seasonal variation in blood pressure with single-pill fixed-dose combinations of angiotensin II receptor blocker/calcium channel blocker and angiotensin II receptor blocker/diuretic in hypertensive patients. J Clin Med Res. 2015;7:802–6.
Kronish IM, Lynch AI, Oparil S, Whittle J, Davis BR, Simpson LM, Krousel-Wood M, Cushman WC, Chang TI, Muntner P. The association between antihypertensive medication nonadherence and visit-to-visit variability of blood pressure: findings from the Antihypertensive and Lipid-lowering Treatment to Prevent Heart Attack Trial. Hypertension. 2016;68:39–45.
Men X, Sun W, Fan F, Zhao M, Huang X, Wang Y, Liu L, Sun W, Peng Q, Qin X, Tang G, Li J, Zhang Y, Cai Y, Hou Y. China Stroke Primary Prevention Trial: visit-to-visit systolic blood pressure variability is an independent predictor of primary stroke in hypertensive patients. J Am Heart Assoc. 2017;6:pii: e004350
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Modesti, P.A., Rapi, S., Rogolino, A. et al. Seasonal blood pressure variation: implications for cardiovascular risk stratification. Hypertens Res 41, 475–482 (2018). https://doi.org/10.1038/s41440-018-0048-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0048-y
This article is cited by
-
Monitoring with wearable devices will clarify the association between indoor temperature and blood pressure
Hypertension Research (2023)
-
Mechanical deconditioning of the heart due to long-term bed rest as observed on seismocardiogram morphology
npj Microgravity (2022)
-
Blood pressure fluctuations and the indoor environment in a highly insulated and airtight model house during the cold winter season
Hypertension Research (2022)
-
Seasonal variation in blood pressure: current evidence and recommendations for hypertension management
Hypertension Research (2021)
-
The relationship between home blood pressure measurement and room temperature in a Japanese general population
Hypertension Research (2021)