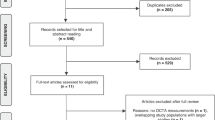
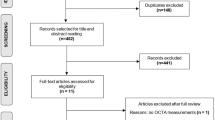
Abstract
Optical coherence tomography angiography (OCT-A) is an ocular imaging technology that has emerged as a non-invasive tool to evaluate retinal microvascular changes in neurodegenerative diseases including Parkinson’s disease (PD) and Alzheimer’s disease. While several studies have reported on the presence of pathologic retinal microvascular alterations in PD, the utility of OCT-A as a biomarker for PD evaluation is still unclear. A systematic review and meta-analysis were performed to explore the current evidence for the role of OCT-A in PD published up until June 2022. PubMed, Scopus, and Web of Science databases were used to systematically identify relevant papers and a meta-analysis was conducted using Stata16 software according to the level of heterogeneity applying a random- or fixed-effect model. Thirteen studies of 925 eyes in the PD group and 1501 eyes in the control group assessing OCT-A findings in PD patients were included. The meta-analyses revealed that the foveal region of PD patients had a significantly lower vessel density in the superficial capillary plexus (SCP) compared to healthy controls but that there were no significant differences in the foveal avascular zone, the SCP in whole, parafoveal, and perifoveal regions, and deep capillary plexus. OCT-A metrics may act as a potential biomarker for a more accurate and early PD diagnosis. Still, the OCT-A algorithms and interchangeability between OCT-A devices require further standardization to draw clinical conclusions regarding their utility.
摘要 摘要
相干光断层扫描血管成像 (Optical coherence tomography angiography, OCT-A) 是一种非侵入性眼部成像技术, 用于评估帕金森病 (Parkinson’s disease, PD) 和阿尔茨海默病等神经退行性疾病的视网膜微血管变化。虽然有几项研究报道了PD中存在视网膜病理性微血管改变, 但OCT-A用于评估PD的作用尚不清楚。我们进行了系统综述和荟萃分析, 检索了截至2022年6月发表的OCT-A在帕金森病中作用的现有证据。使用PubMed、Scopus和Web of Science数据库系统地检索相关文献, 并使用Stata16软件根据随机或固定效应模型的异质性水平进行meta分析。纳入13项研究, 包括PD组925只眼和对照组1501只眼, 评估PD患者OCT-A发现。荟萃分析显示, 与健康对照组相比, PD患者的中心凹区浅层毛细血管丛 (superficial capillary plexus, SCP) 的血管密度显著降低, 但在中心凹无血管区、整个、中心凹旁和中心凹周围区域的SCP和深层毛细血管丛中无统计学差异。OCT-A在PD早期精确诊断中可能作为一个潜在的生物标志物。但是OCT-A算法和OCT-A设备之间的互换性需要进一步标准化, 以得出关于其临床实用性的结论。
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Change history
15 September 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41433-023-02691-w
References
Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912.
Chen RC, Chang SF, Su CL, H. Chen TH, Yen MF, Wu HM, et al. Prevalence, incidence, and mortality of PD. Neurology 2001;57:1679.
Lotharius J, Brundin P. Pathogenesis of Parkinson’s disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci. 2002;3:932–42.
Pfeiffer RF. Non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord 2016;22:S119–22.
Guo L, Normando EM, Shah PA, De Groef L, Cordeiro MF. Oculo-visual abnormalities in Parkinson’s disease: Possible value as biomarkers. Mov Disord. 2018;33:1390–406.
Mammadova N, Summers CM, Kokemuller RD, He Q, Ding S, Baron T, et al. Accelerated accumulation of retinal α-synuclein (pSer129) and tau, neuroinflammation, and autophagic dysregulation in a seeded mouse model of Parkinson’s disease. Neurobiol Dis. 2019;121:1–16.
Lin CW, Lai TT, Chen SJ, Lin CH. Elevated α-synuclein and NfL levels in tear fluids and decreased retinal microvascular densities in patients with Parkinson’s disease. Geroscience. 2022;44:1551–62.
Williams DR, Litvan I. Parkinsonian syndromes. Continuum (Minneap Minn) 2013;19:1189–212.
Postuma RB, Aarsland D, Barone P, Burn DJ, Hawkes CH, Oertel W, et al. Identifying prodromal Parkinson’s disease: pre-motor disorders in Parkinson’s disease. Mov Disord. 2012;27:617–26.
Zhang C, Wu B, Wang X, Chen C, Zhao R, Lu H, et al. Vascular, flow and perfusion abnormalities in Parkinson’s disease. Parkinsonism Relat Disord. 2020;73:8–13.
Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37:56–74.
Guan J, Pavlovic D, Dalkie N, Waldvogel HJ, O’Carroll SJ, Green CR, et al. Vascular degeneration in Parkinson’s disease. Brain Pathol. 2013;23:154–64.
Wan Y, Hu W, Gan J, Song L, Wu N, Chen Y, et al. Exploring the association between Cerebral small-vessel diseases and motor symptoms in Parkinson’s disease. Brain Behav. 2019;9:e01219.
London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol. 2013;9:44–53.
Asanad S, Mohammed I, Sadun AA, Saeedi OJ. OCTA in neurodegenerative optic neuropathies: emerging biomarkers at the eye-brain interface. Ther Adv Ophthalmol. 2020;12:2515841420950508.
Pellegrini M, Vagge A, Ferro Desideri LF, Bernabei F, Triolo G, Mastropasqua R, et al. Optical coherence tomography angiography in neurodegenerative disorders. J Clin Med. 2020;9:1706.
Tsokolas G, Tsaousis KT, Diakonis VF, Matsou A, Tyradellis S. Optical coherence tomography angiography in neurodegenerative diseases: a review. Eye Brain. 2020;12:73–87.
Rifai OM, McGrory S, Robbins CB, Grewal DS, Liu A, Fekrat S, et al. The application of optical coherence tomography angiography in Alzheimer’s disease: A systematic review. Alzheimers Dement. 2021;13:e12149.
Christou EE, Asproudis I, Asproudis C, Giannakis A, Stefaniotou M, Konitsiotis S. Macular microcirculation characteristics in Parkinson’s disease evaluated by OCT-Angiography: a literature review. Semin Ophthalmol. 2022;37:399–407.
Mardin CY, Hosari S. Optical coherence tomography angiography in neuronal diseases: Preliminary findings. Ophthalmologe 2019;116:714–21.
Zhou WC, Tao JX, Li J. Optical coherence tomography measurements as potential imaging biomarkers for Parkinson’s disease: A systematic review and meta-analysis. Eur J Neurol. 2021;28:763–74.
Zhang Y, Yang L, Gao Y, Zhang D, Tao Y, Xu H, et al. Choroid and choriocapillaris changes in early-stage Parkinson’s disease: a swept-source optical coherence tomography angiography-based cross-sectional study. Alzheimers Res Ther. 2022;14:116.
Satue M, Castro L, Vilades E, Cordon B, Errea JM, Pueyo A, et al. Ability of Swept-source OCT and OCT-angiography to detect neuroretinal and vasculature changes in patients with Parkinson’s disease and essential tremor. Eye. 2022. https://doi.org/10.1038/s41433-022-02112-4. Online ahead of print.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89.
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591–601.
Kwapong WR, Ye H, Peng C, Zhuang X, Wang J, Shen M, et al. Retinal microvascular impairment in the early stages of Parkinson’s disease. Invest Ophthalmol Vis Sci. 2018;59:4115–22.
Grewal DS, Fekrat S, Fine HF. Is OCT angiography useful in neurodegenerative diseases? Ophthalmic Surg Lasers Imaging Retin. 2019;50:269–73.
Zafar S, McCormick J, Giancardo L, Saidha S, Abraham A, Channa R. Retinal imaging for neurological diseases: a window into the brain. Int Ophthalmol Clin. 2019;59:137–54.
Shi C, Chen Y, Kwapong WR, Tong Q, Wu S, Zhou Y, et al. Characterization by fractal dimension analysis of the retinal capillary network in parkinson disease. Retina 2020;40:1483–91.
Zou J, Liu K, Li F, Xu Y, Shen L, Xu H. Combination of optical coherence tomography (OCT) and OCT angiography increases diagnostic efficacy of Parkinson’s disease. Quant Imaging Med Surg. 2020;10:1930–9.
Lin JB, Apte RS. Seeing Parkinson’s disease in the retina. JAMA Ophthalmol. 2021;139:189–90.
Murueta-Goyena A, Barrenechea M, Erramuzpe A, Teijeira-Portas S, Pengo M, Ayala U, et al. Foveal remodeling of retinal microvasculature in Parkinson’s disease. Front Neurosci. 2021;15:708700.
Rascunà C, Cicero CE, Chisari CG, Russo A, Giuliano L, Castellino N, et al. Retinal thickness and microvascular pathway in Idiopathic Rapid eye movement sleep behaviour disorder and Parkinson’s disease. Parkinsonism Relat Disord. 2021;88:40–5.
Zhou M, Wu L, Hu Q, Wang C, Ye J, Chen T, et al. Visual impairments are associated with retinal microvascular density in patients with Parkinson’s disease. Front Neurosci. 2021;15:718820.
Xu B, Wang X, Guo J, Xu H, Tang B, Jiao B, et al. Retinal microvascular density was associated with the clinical progression of Parkinson’s disease. Front Aging Neurosci. 2022;14:818597.
Lauermann JL, Sochurek JAM, Plöttner P, Alten F, Kasten M, Prasuhn J, et al. Applicability of optical coherence tomography angiography (OCTA) imaging in Parkinson’s disease. Sci Rep. 2021;11:5520.
Robbins CB, Thompson AC, Bhullar PK, Koo HY, Agrawal R, Soundararajan S, et al. Characterization of retinal microvascular and choroidal structural changes in Parkinson disease. JAMA Ophthalmol. 2021;139:182–8.
Robbins CB, Grewal DS, Thompson AC, Soundararajan S, Yoon SP, Polascik BW, et al. Identifying peripapillary radial capillary plexus alterations in Parkinson’s disease using OCT Angiography. Ophthalmol Retin. 2022;6:29–36.
Zhang Y, Zhang D, Gao Y, Yang L, Tao Y, Xu H, et al. Retinal flow density changes in early-stage Parkinson’s disease investigated by swept-source optical coherence tomography angiography. Curr Eye Res. 2021;46:1886–91.
Rascunà C, Russo A, Terravecchia C, Castellino N, Avitabile T, Bonfiglio V, et al. Retinal thickness and microvascular pattern in early Parkinson’s disease. Front Neurology. 2020;11:533375.
Li Y, Wang X, Zhang Y, Zhang P, He C, Li R, et al. Retinal microvascular impairment in Parkinson’s disease with cognitive dysfunction. Parkinsonism Relat Disord. 2022;98:27–31.
Lu Y, Wang JC, Zeng R, Katz R, Vavvas DG, Miller JW, et al. Quantitative comparison of microvascular metrics on three optical coherence tomography angiography devices in chorioretinal disease. Clin Ophthalmol. 2019;13:2063–9.
Munk MR, Giannakaki-Zimmermann H, Berger L, Huf W, Ebneter A, Wolf S, et al. OCT-angiography: A qualitative and quantitative comparison of 4 OCT-A devices. PLoS One. 2017;12:e0177059.
Paul G, Elabi OF. Microvascular changes in Parkinson’s disease- focus on the neurovascular unit. Front Aging Neurosci. 2022;14:853372.
Ortuño-Lizarán I, Beach TG, Serrano GE, Walker DG, Adler CH, Cuenca N. Phosphorylated α-synuclein in the retina is a biomarker of Parkinson’s disease pathology severity. Mov Disord. 2018;33:1315–24.
Elabi O, Gaceb A, Carlsson R, Padel T, Soylu-Kucharz R, Cortijo I, et al. Human α-synuclein overexpression in a mouse model of Parkinson’s disease leads to vascular pathology, blood-brain barrier leakage and pericyte activation. Sci Rep. 2021;11:1120.
Bodis-Wollner I. Foveal vision is impaired in Parkinson’s disease. Parkinsonism Relat Disord. 2013;19:1–14.
Spund B, Ding Y, Liu T, Selesnick I, Glazman S, Shrier EM, et al. Remodeling of the fovea in Parkinson disease. J Neural Transm. 2013;120:745–53.
Huang L, Zhang D, Ji J, Wang Y, Zhang R. Central retina changes in Parkinson’s disease: a systematic review and meta-analysis. J Neurol. 2021;268:4646–54.
Chen JJ, AbouChehade JE, Iezzi R Jr, Leavitt JA, Kardon RH. Optical coherence angiographic demonstration of retinal changes from chronic optic neuropathies. Neuroophthalmology. 2017;41:76–83.
Deng Y, Jie C, Wang J, Liu Z, Li Y, Hou X. Evaluation of retina and microvascular changes in the patient with Parkinson’s disease: A systematic review and meta-analysis. Front Med. 2022;9:957700.
Powell A, Muller AJ, O’Callaghan C, Sourty M, Shine JM, Lewis SJG. Dopamine and functional connectivity in patients with Parkinson’s disease and visual hallucinations. Mov Disord. 2020;35:704–5.
Garcia-Martin E, Larrosa JM, Polo V, Satue M, Marques ML, Alarcia R, et al. Distribution of retinal layer atrophy in patients with Parkinson’s disease and association with disease severity and duration. Am J Ophthalmol. 2014;157:470–8.e2.
Yu DY, Cringle SJ, Yu PK, Balaratnasingam C, Mehnert A, Sarunic MV, et al. Retinal capillary perfusion: Spatial and temporal heterogeneity. Prog Retin Eye Res. 2019;70:23–54.
Price DL, Rockenstein E, Mante M, Adame A, Overk C, Spencer B, et al. Longitudinal live imaging of retinal α-synuclein::GFP deposits in a transgenic mouse model of Parkinson’s Disease/Dementia with Lewy Bodies. Sci Rep. 2016;6:29523.
Lu Y, Wang JC, Cui Y, Zhu Y, Zeng R, Lu ES, et al. A quantitative comparison of four optical coherence tomography angiography devices in healthy eyes. Graefes Arch Clin Exp Ophthalmol. 2021;259:1493–501.
Corvi F, Cozzi M, Barbolini E, Nizza D, Belotti M, Staurenghi G, et al. Comparison between several optical coherence tomography angiography devices and indocyanine green angiography of choroidal neovascularization. Retina 2020;40:873–80.
Corvi F, Pellegrini M, Erba S, Cozzi M, Staurenghi G, Giani A. Reproducibility of vessel density, fractal dimension, and Foveal Avascular zone using 7 different optical coherence tomography angiography devices. Am J Ophthalmol. 2018;186:25–31.
Mihailovic N, Brand C, Lahme L, Schubert F, Bormann E, Eter N, et al. Repeatability, reproducibility and agreement of foveal avascular zone measurements using three different optical coherence tomography angiography devices. PLoS One. 2018;13:e0206045.
Ranjbar M, Plöttner P, Sochurek JAM, Lauermann JL, Alten F, Prasuhn J, et al. The impact of motion artifacts on quantitative optical coherence tomography angiography analysis in Parkinson’s disease. Parkinsonism Relat Disord. 2022;95:57–8.
Xiao H, Liu X, Liao L, Tan K, Ling Y, Zhong Y. Reproducibility of Foveal Avascular zone and superficial macular retinal vasculature measurements in healthy eyes determined by two different scanning protocols of optical coherence tomography angiography. Ophthalmic Res. 2020;63:244–51.
Author information
Authors and Affiliations
Contributions
Author contributions included conception and study design (SM and MAS), article screening (AJ and MAS and SM), data collection or acquisition (SSZ, MAS, and SM), statistical analysis (MAS and SM), interpretation of results (MAS and SM and FR), drafting the manuscript work or revising it critically for important intellectual content (MAS, SM, FR, MG, GY, IS, SJ, and RS) and approval of the final version to be published and agreement to be accountable for the integrity and accuracy of all aspects of the work (MAS, SM, FR, MG, GY, IS, SJ, and RS).
Corresponding author
Ethics declarations
Competing interests
Authors declare no conflicts of interest except Dr. Rishi P. Singh who is a consultant for Genentech, Regeneron, Alcon, Bausch and Lomb, Asclepix, Gyroscope, Novartis.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised:. In the sub-section ‘Density measurements in deep capillary plexus’, the following sentence “Meta-analysis of these three studies also did not reveal any significant changes (MD, −0.61; 95% CI [−1.78 to 0.56]; P-value = 0.30; I² = 0.00)” was corrected to read “Meta-analysis of these two studies (26,40) also did not reveal any significant changes (MD, −0.61; 95% CI [−1.78 to 0.56]; P-value = 0.30; I² = 0.00)”. Additionally, the reference numbers in the tables 1, 2, 3, as well as the supplementary tables s1 and s2 have been corrected.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salehi, M.A., Rezagholi, F., Mohammadi, S. et al. Optical coherence tomography angiography measurements in Parkinson’s disease: A systematic review and meta-analysis. Eye 37, 3145–3156 (2023). https://doi.org/10.1038/s41433-023-02483-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02483-2
This article is cited by
-
Translating discoveries as novel biomarkers and interventions in ophthalmology
Journal of Translational Medicine (2023)
-
Methodologic lessons from published systematic reviews
Eye (2023)