Abstract
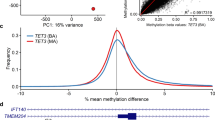
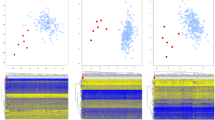
The challenges and ambiguities in providing an accurate diagnosis for patients with neurodevelopmental disorders have led researchers to apply epigenetics as a technique to validate the diagnosis provided based on the clinical examination and genetic testing results. Genome-wide DNA methylation analysis has recently been adapted for clinical testing of patients with genetic neurodevelopmental disorders. In this paper, preliminary data demonstrating a DNA methylation signature for Renpenning syndrome (RENS1 – OMIM 309500), which is an X-linked recessive neurodevelopmental disorder caused by variants in polyglutamine-binding protein 1 (PQBP1) is reported. The identified episignature was then utilized to construct a highly sensitive and specific binary classification model. Besides providing evidence for the existence of a DNA methylation episignature for Renpenning syndrome, this study increases the knowledge of the molecular mechanisms related to the disease. Moreover, the availability of more subjects in future may facilitate the establishment of an episignature that can be utilized for diagnosis in a clinical setting and for reclassification of variants of unknown clinical significance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Some of the datasets used in this study are publicly available and may be obtained from gene expression omnibus (GEO) using the following accession numbers. GEO: GSE116992, GSE66552, GSE74432, GSE97362, GSE116300, GSE95040, GSE 104451, GSE125367, GSE55491, GSE108423, GSE116300, GSE 89353, GSE52588, GSE42861, GSE85210, GSE87571, GSE87648, GSE99863, and GSE35069. These include DNA methylation data from patients with Kabuki syndrome, Sotos syndrome, CHARGE syndrome, immunodeficiency-centromeric instability-facial anomalies (ICF) syndrome, Williams-Beuren syndrome, Chr7q11.23 duplication syndrome, BAFopathies, Down syndrome, a large cohort of unresolved subjects with developmental delays and congenital abnormalities, and also several large cohorts of DNA methylation data from the general population. The rest of the data including the RENS1 samples are not available due to the restrictions of the ethics approval. The variants are publicly available on ClinVar, with accession numbers SCV002525441.1- SCV002525447.1.
References
Christianson A, Howson CP, Modell B. March of Dimes. Global report on birth defect. The hidden toll of dying and disabled children. New York: White Plains; 2006.
Sadikovic B, Aref-Eshghi E, Levy MA, Rodenhiser D. DNA methylation signatures in mendelian developmental disorders as a diagnostic bridge between genotype and phenotype. Epigenomics 2019;11:563–75.
Bjornsson HT. The Mendelian disorders of the epigenetic machinery. Genome Res. 2015;25:1473–81.
Fahrner J, Bjornsson H. Mendelian disorders of the epigenetic machinery: Tipping the balance of chromatin states. Ann Rev Genom Hum Genet. 2014;15:269–93.
Aref-Eshghi E, Kerkhof J, Pedro VP, Barat-Houari M, Ruiz-Pallares N, Andrau JC, et al. Evaluation of DNA methylation episignatures for diagnosis and phenotype correlations in 42 Mendelian neurodevelopmental disorders. Am J Hum Genet. 2020;106:356–70.
Sadikovic B, Levy MA, Kerkhof J, Aref-Eshghi E, Schenkel L, Stuart A, et al. Clinical epigenomics: genome-wide DNA methylation analysis for the diagnosis of Mendelian disorders. Genet Med. 2021;23:1–10.
Levy MA, McConkey H, Kerkhof J, Barat-Houari M, Bargiacchi S, Biamino E, et al. Novel diagnostic DNA methylation episignatures expand and refine the epigenetic landscapes of Mendelian disorders. Hum Genet Genom Adv. 2021;3:100075.
Renpenning H, Gerrard JW, Zaleski WA, Tabata T. Familial sex-linked mental retardation. Can Med Assoc J. 1962;87:954–6.
Lenski C, Abidi F, Meindl A, Gibson A, Platzer M, Kooy RF, et al. Novel truncating mutations in the polyglutamine tract binding protein 1 gene (PQBP1) cause Renpenning syndrome and X-linked mental retardation in another family with microcephaly. Am J Hum Genet. 2004;74:777–80.
Stevenson RE, Arena JF, Ouzts E, Gibson A, Shokeir MHK, Vnencak-Jones C, et al. Renpenning syndrome maps to Xp11. Am J Hum Genet. 1998;62:1092–101.
Sutherland GR, Gedeon AK, Haan EA, Woodroffe P, Mulley JC. Linkage studies with the gene for an X-linked syndrome of mental retardation, microcephaly and spastic diplegia (MRX2). Am J Med Genet. 1988;30:493–508.
Lubs H, Abidi FE, Echeverri R, Holloway L, Meindl A, Stevenson RE, et al. Golabi-Ito-Hall syndrome results from a missense mutation in the WW domain of the PQBP1 gene. J Med Genet. 2006;43:1–4.
Porteous M, Johnson H, Burn J, Curtis A, Lindsay S, Bhattacharya S, et al. A new mental retardation syndrome mapping to the pericentromeric region of the X-chromosome. Am J Hum Genet. 1992;51:A106.
Hamel BCJ, Mariman ECM, Van Beersum SEC, Schoonbrood-Lenssen AMJ, Ropers HH. Mental retardation, congenital heart defect, cleft palate, short stature, and facial anomalies: A new X-linked multiple congenital anomalies/mental retardation syndrome: Clinical description and molecular studies. Am J Med Genet. 1994;51:591–7.
Deqaqi SC, N’Guessan M, Forner J, Sbiti A, Beldjord C, Chelly J, et al. A gene for non-specific X-linked mental retardation (MRX55) is located in Xp11. Ann Genet. 1998;41:11–6.
Stevenson RE, Bennett CW, Abidi F, Kleefstra T, Porteous M, Simensen RJ, et al. Renpenning syndrome comes into focus. Am J Med Genet. 2005;134 A:415–21.
Germanaud D, Rossi M, Bussy G, Gérard D, Hertz-Pannier L, Blanchet P, et al. The Renpenning syndrome spectrum: New clinical insights supported by 13 new PQBP1-mutated males. Clin Genet. 2011;79:225–35.
Masih S, Moirangthem A, Phadke SR. Renpenning syndrome in an Indian patient. Am J Med Genet Part A. 2020;182:293–5.
Kaymakçalan H, Ercan-Şençiçek AG, Cebeci AN, Dong W, Yalçın ASY. A rare etiology of tetralogy of Fallot with pulmonary atresia: Renpenning syndrome. Anatol J Cardiol. 2022;26:149–50.
Jeong H-I, Yang A, Kim J, Jang J-H, Cho SY, Jin D-K. First Korean case of Renpenning syndrome with novel mutation in PQBP1 diagnosed by targeted exome sequencing, and literature review. Ann Clin Lab Sci. 2018;48:522–7.
Cho RY, Peñaherrera MS, Du Souich C, Huang L, Mwenifumbo J, Nelson TN, et al. Renpenning syndrome in a female. Am J Med Genet Part A. 2020;182:498–503.
Rees M, Gorba C, De Chiara C, Bui TTT, Garcia-Maya M, Drake AF, et al. Solution model of the intrinsically disordered polyglutamine tract-binding protein-1. Biophys J. 2012;102:1608–16.
Rahman SK, Okazawa H, Chen YW. Frameshift PQBP-1 mutants K192Sfs*7 and R153Sfs*41 implicated in X-linked intellectual disability form stable dimers. J Struct Biol. 2019;206:305–13.
Mizuguchi M, Obita T, Serita T, Kojima R, Nabeshima Y, Okazawa H. Mutations in the PQBP1 gene prevent its interaction with the spliceosomal protein U5-15kD. Nat Commun. 2014;5:1–5.
Iwasaki Y, Thomsen GH. The splicing factor PQBP1 regulates mesodermal and neural development through FGF signaling. Dev. 2014;141:3740–51.
Tapia VE, Nicolaescu E, McDonald CB, Musi V, Oka T, Inayoshi Y, et al. Y65C missense mutation in the WW domain of the Golabi-Ito-Hall syndrome protein PQBP1 affects its binding activity and deregulates pre-mRNA splicing. J Biol Chem. 2010;285:19391–401.
Wan D, Zhang ZC, Zhang X, Li Q, Han J. X chromosome-linked intellectual disability protein PQBP1 associates with and regulates the translation of specific mRNAs. Hum Mol Genet. 2015;24:4599–614.
Wang Q, Moore MJ, Adelmant G, Marto JA, Silver PA. PQBP1, a factor linked to intellectual disability, affects alternative splicing associated with neurite outgrowth. Genes Dev. 2013;27:615–26.
Sadikovic B, Levy MA, Aref-Eshghi E. Functional annotation of genomic variation: DNA methylation episignatures in neurodevelopmental Mendelian disorders. Hum Mol Genet. 2020;29:R27–32.
Aref-Eshghi E, Bend EG, Hood RL, Schenkel LC, Carere DA, Chakrabarti R, et al. BAFopathies’ DNA methylation epi-signatures demonstrate diagnostic utility and functional continuum of Coffin–Siris and Nicolaides–Baraitser syndromes. Nat Commun. 2018;9:4885.
Aref-Eshghi E, Rodenhiser DI, Schenkel LC, Lin H, Skinner C, Ainsworth P, et al. Genomic DNA methylation signatures enable concurrent diagnosis and clinical genetic variant classification in neurodevelopmental syndromes. Am J Hum Genet. 2018;102:156–74.
Aref-Eshghi E, Bend EG, Colaiacovo S, Caudle M, Chakrabarti R, Napier M, et al. Diagnostic utility of genome-wide DNA methylation testing in genetically unsolved individuals with suspected hereditary conditions. Am J Hum Genet. 2019;104:685–700.
Aryee MJ, Jaffe AE, Corrada-Bravo H, Ladd-Acosta C, Feinberg AP, Hansen KD, et al. Minfi: A flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 2014;30:1363–9.
Pidsley R, Wong CCY, Volta M, Lunnon K, Mill J, Schalkwyk LC. A data-driven approach to preprocessing Illumina 450K methylation array data. BMC Genom. 2013;14:1–10.
Ho DE, Imai K, King G, Stuart EA. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal. 2007;15:199–236.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47.
Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinforma. 2012;13:1–16.
Peters TJ, Buckley MJ, Statham AL. De novo identification of differentially methylated regions in the human genome. Epigenet Chromatin. 2015;8:1–16.
Phipson B, Maksimovic J, Oshlack A. MissMethyl: An R package for analyzing data from Illumina’s HumanMethylation450 platform. Bioinformatics 2016;32:286–8.
Lv X, Jiang H, Li B, Liang Q, Wang S, Zhao Q, et al. The crucial role of Atg5 in cortical neurogenesis during early brain development. Sci Rep. 2014;4:1–9.
Kim M, Sandford E, Gatica D, Qiu Y, Liu X, Zheng Y, et al. Mutation in ATG5 reduces autophagy and leads to ataxia with developmental delay. Elife. 2016;5:e12245.
Choufani S, Cytrynbaum C, Chung BHY, Turinsky AL, Grafodatskaya D, Chen YA, et al. NSD1 mutations generate a genome-wide DNA methylation signature. Nat Commun. 2015;6:1–7.
Aref-Eshghi E, Schenkel LC, Lin H, Skinner C, Ainsworth P, Paré G, et al. The defining DNA methylation signature of Kabuki syndrome enables functional assessment of genetic variants of unknown clinical significance. Epigenetics 2017;12:923–33.
Schenkel LC, Kernohan KD, McBride A, Reina D, Hodge A, Ainsworth PJ, et al. Identification of epigenetic signature associated with alpha thalassemia/mental retardation X-linked syndrome. Epigenet Chromatin. 2017;10:1–11.
Golabi M, Ito M, Hall BD. A new X-linked multiple congenital anomalies/mental retardation syndrome. Am J Med Genet. 1984;17:367–74.
Mittendorf K, Deatherage C, Ohi M, Sanders C. Tailoring of membrane proteins by alternative splicing of pre-mRNA. Biochemistry 2012;51:5541–56.
Sural-Fehr T, Bongarzone E. How membrane dysfunction influences neuronal survival pathways in sphingolipid storage disorders. J Neurosci Res. 2016;94:1042–8.
Sudol M, McDonald CB, Farooq A. Molecular Insights into the WW domain of the Golabi-Ito-Hall Syndrome Protein PQBP1. FEBS Lett. 2012;586:2795–9.
Haghshenas S, Levy MA, Kerkhof J, Aref-Eshghi E, McConkey H, Balci T, et al. Detection of a dna methylation signature for the intellectual developmental disorder, x-linked, syndromic, armfield type. Int J Mol Sci. 2021;22:1–13.
Lee YR, Khan K, Armfield-Uhas K, Srikanth S, Thompson NA, Pardo M, et al. Mutations in FAM50A suggest that Armfield XLID syndrome is a spliceosomopathy. Nat Commun. 2020;11:3698.
Acknowledgements
We would like to thank the families for their contribution to Greenwood Genetic Center’s (GGC’s) study of X-linked intellectual disabilities. We also appreciate Anna Crockett’s assistance in preparing revisions to the revising of the paper. Dedicated to the memory of Ethan Francis Schwartz, 1996–1998.
Funding
This work was funded in part by the government of Canada through Genome Canada and the Ontario Genomics Institute (OGI-188) awarded to BS, NIH grant HD-026202 to CES, the South Carolina Department of Disabilities and Special Needs, and the Greenwood Genetic Center Foundation.
Author information
Authors and Affiliations
Contributions
Conceptualization: SH, BS, CES; Data curation: SH, JK; Formal analysis: SH, MAL, RR; Funding acquisition: BS, CES; Investigation: SH, MAL, JK, CDS, RCC; Methodology: SH, MAL, RR; Project administration: HM, BS, CES; Resources: JK, CDS, MLT, RES, BS, CES; Software: SH, MAL, RR; Supervision: BS, CES; Validation: SH, AF, PB; Visualization: SH; Writing-original draft: SH, AF; Writing-review and editing: PB, HM, RES, BS, CES.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential competing interest. CES was previously affiliated with GGC, which provides commercial diagnostic services using EpiSign. This study however, was initiated after his retirement from GGC in collaboration with BS. Some of the genome-wide data was generated at GGC on a research basis at the request of CES. Thus, it is not linked to the commercial diagnostic services at GGC.
Ethics approval
All of the samples and records were de-identified. The study protocol has been approved by the Western University Research Ethics Board (REB 106302), and the IRB of Self Regional Healthcare. Informed consent was obtained by physicians for use of the clinical information of the described patients.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haghshenas, S., Foroutan, A., Bhai, P. et al. Identification of a DNA methylation signature for Renpenning syndrome (RENS1), a spliceopathy. Eur J Hum Genet 31, 879–886 (2023). https://doi.org/10.1038/s41431-023-01313-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-023-01313-z
This article is cited by
-
2023 in the European Journal of Human Genetics
European Journal of Human Genetics (2024)
-
Molecular consequences of PQBP1 deficiency, involved in the X-linked Renpenning syndrome
Molecular Psychiatry (2023)
-
A new impact factor for EJHG in 2022
European Journal of Human Genetics (2023)