Abstract
Homologous recombination and chromosome segregation in meiosis rely on the timely expression of two splice variants of the endonuclease SPO11, named α and β, which respectively skip or include exon 2. However, in spite of its physiological importance, the mechanism underlying Spo11 alternative splicing in meiosis is still unknown. By screening the activity of factors that are predicted to bind the alternatively spliced region of Spo11, we identified hnRNPH as a key regulator of SPO11α splicing in mouse spermatocytes. Although hnRNPH was not upregulated in meiosis concomitantly with the switch in splicing, its recruitment to Spo11 pre-mRNA was favored by selective modulation of RNA polymerase II (RNAPII) phosphorylation and processivity in proximity of exon 2. The hnRNPH binding sites were localized near those of splicing factors that promote SPO11β splicing, suggesting that hnRNPH favors exon 2 skipping by competing out positive regulators. Indeed, hnRNPH binds proximal to a consensus motif for Sam68, a positive regulator of SPO11β splicing in vitro and in vivo, and it interferes with Sam68 binding to the Spo11 pre-mRNA. Thus, our work reveals that modulation of RNAPII dynamics in concert with hnRNPH recruitment exerts a combinatorial control of the timely regulated Spo11 splicing during meiosis.
Similar content being viewed by others
Introduction
Alternative splicing (AS) of pre-mRNAs is a powerful combinatorial mechanism that allows production of protein isoforms with different functions from each gene1,2. AS modularity allows expansion of the coding potential of the genome and promotes plasticity in its utilization1. Splicing reactions are orchestrated by a ribonucleoprotein complex called “spliceosome”, which recognizes exon–intron junctions, excises introns and ligates exons3. The lack of stringent consensus sequences at splice junctions in higher eukaryotes allows flexibility in their recognition. Numerous RNA-binding proteins (RBPs) act as splicing factors by interacting with the spliceosome, and reinforce or weaken recognition of exon–intron junctions. The interplay between these antagonistic splicing factors determines the choice of regulated exons by the spliceosome and causes heterogeneity in pre-mRNA processing1,3,4. In addition, AS is modulated by the rate of transcription and by epigenetic marks that decorate exons subject to regulation5,6. As a consequence, changes in the expression and/or in the activity of any of the factors contributing to these processes can selectively influence AS regulation. In particular, the elongation rate of the RNA polymerase II (RNAPII) is a key factor in co-transcriptional selection of regulated exons. A fast elongation rate makes more splice sites concomitantly available within a pre-mRNA, allowing competition between them and generally favoring stronger splice sites, whereas a slow rate of elongation promotes inclusion of weak exons5,7. However, genome-wide analyses indicated that several exons were also preferentially skipped by slowing down the RNAPII8. Such effect could rely on preferential recruitment of repressive splicing factors by the slow polymerase in the proximity of the regulated exon, as demonstrated for the CFTR gene9. Nevertheless, when and how such specific coordination is achieved under physiological situations remains largely unknown.
Testis is the organ displaying the highest abundance of splice variants10. In particular, meiotic spermatocytes display exceptional transcriptional and splicing diversity10,11,12. AS flexibility is exploited by meiotic cells to produce protein variants uniquely required for the peculiar processes involved in germ cell differentiation13,14, but also to dictate the timing of their expression12. An interesting example in this sense is offered by the Spo11 gene, encoding the essential endonuclease that establishes DNA double strand breaks (DSBs) and initiates homologous recombination in meiosis15,16. The Spo11 gene encodes for two main protein isoforms (SPO11α and β) which differ for exon 2 skipping (α) or inclusion (β). Early meiotic spermatocytes synthesize primarily SPO11β, whereas SPO11α becomes predominant in late meiosis17. Notably, the timing of Spo11 AS parallels that of DSB formation during meiosis, with a first wave in leptotene/zygotene spermatocytes that affects autosomal chromosomes and a delayed one that preferentially marks the sex chromosomes in late pachytene. Transgenic mice expressing only SPO11β were fully competent in establishing the first DSB wave, but late foci in sex chromosomes, which appear in concomitance with SPO11α splicing, were suppressed, leading to inefficient X–Y pairing and recombination18. Thus, the Spo11 splice variants appear to operate on distinct subsets of meiotic DSBs that are both essential for gamete differentiation18. However, in spite of its physiological importance, the mechanism(s) underlying Spo11 AS during meiosis are still unknown.
Herein, we describe a novel mechanism involved in the regulation of Spo11 AS. We found that hnRNPH strongly induces SPO11α splicing. Interestingly, hnRNPH was not upregulated in pachytene spermatocytes when the switch in splicing choice occurs. However, splicing regulation was paralleled by a decrease in the RNAPII elongation rate within the Spo11 transcription, which promoted hnRNPH recruitment and exon 2 skipping. Mechanistically, hnRNPH competed for binding and splicing of the Spo11 pre-mRNA with Sam68, a splicing factor whose ablation causes meiotic defects19. Thus, our work uncovers a fine-tuned combinatorial mechanism underlying the timely regulation of Spo11 splicing during meiosis.
Results
HnRNPH is a strong modulator of SPO11α splicing
To determine the timing of Spo11 splicing regulation, we isolated germ cells from CD1 mice during the first spermatogenic wave (8–25 postnatal day, P), when fronts of germ cells almost synchronously enter into specific differentiation stages20. As observed in another strain17, SPO11β was the predominant variant expressed at P8 and P14 (Fig. 1a; Supplementary Fig. S1A), when testis is mostly populated by mitotic spermatogonia and early meiotic spermatocytes20, respectively. By contrast, SPO11α becomes the main variant at P18 and P25 (Fig. 1a; Supplementary Fig. S1A), when the most abundant cells are meiotic spermatocytes20. Treatment with the RNAPII inhibitor flavopiridol (FPD) to block transcription12 showed no significant changes in the stability of SPO11α and SPO11β mRNAs at these stages (Fig. 1b–d). These results suggest that a shift in Spo11 splicing regulation occurs between P14 and P18.
a RT-PCR analysis of endogenous SPO11 mRNA in total germ cells extracted from testis at different ages (P) using primers flanking exon 2 that distinguish between the α and β variants. The bar graph represents densitometric analyses of the assay (SPO11α/SPO11β ratio; mean ± SD; n = 3; *P ≤ 0.05, **P ≤ 0.01, unpaired t test). b Representative RT-PCR analysis of three experiment of endogenous genes in total germ cells from P14 and P18 mice treated or not with 1 μM Flavopiridol (FPD) for 6 h. The first row represent Spo11 alternative splicing, the second row represent a portion of the Spo11 pre-mRNA (intron 7–exon 8 region), which is affected by transcriptional inhibition. The Jun gene was used as control of genes with high turnover whereas the 18S ribosomal RNA was used as normalizer. c, d Bar graph represents quantitative real-time PCR (qPCR) analysis of the level of SPO11α (c) and SPO11β (d) relative to Spo11 total in total germ cells from P14 and P18 mice treated or not with 1 μM FPD for 6 h (mean ± SD; n = 3; ns not significant, unpaired t test).
We accurately staged male germ cells by monitoring assembly of the synaptonemal complex in chromosome spreads at P14 and P1821. Pachynema represents the longest stage of prophase I and early pachytene cells can be distinguished by the lack of expression of the testis-specific histone H1t, which becomes detectable in mid- and late-pachytene cells20. Co-staining of chromosome spreads for the synaptonemal complex protein SCP3 and H1t did not reveal major changes in the population of meiotic spermatocytes between P14 and P18 (Supplementary Fig. S1B). Thus, changes in Spo11 AS do not reflect a clear transition in meiosis.
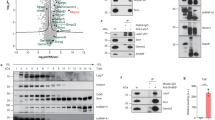
Next, we searched for factors that could promote SPO11α splicing. Analysis of binding sites within exon 2 and flanking intronic regions (100 base pair, bp) using the SpliceAid2 tool (http://193.206.120.249/splicing_tissue.html) identified several splicing factors potentially involved in Spo11 AS in both human and mouse (Fig. 2a; Supplementary Fig. S2A). To test their effect on Spo11 splicing, we constructed a minigene encompassing the genomic region from exon 1 to exon 3 (Fig. 2b). Splicing assays in human HEK293T cells, which do not express SPO11, indicated that the minigene yields both Spo11 variants with a ratio comparable to that of P14 testis (Fig. 2c). Analysis of the predicted factors, as well as of other members of the SR and hnRNP families and of some tissue-specific splicing factors, revealed that hnRNPF and H were the strongest inducers of SPO11α splicing (Fig. 2d, e; Supplementary Fig. S2B), whereas SRSF1, SRSF3, ETR-3, hnRNPA1/A2, RBM11, PTBP1/2 elicited a much milder effect. On the other hand, TRA2α/β, Sam68, SLM2, and hnRNPK enhanced splicing of SPO11β (Fig. 2d, e; Supplementary Fig. S2B). To test if differential expression of these splicing factors could account for the switch between P14 and P18, we performed Western blot analyses on germ cell extracts (Fig. 2f; Supplementary Fig. S2C). Remarkably, none of the tested factors displayed changes in expression compatible with their effect on Spo11 AS, as most were unchanged between P14 and P18 (i.e., Sam68, hnRNPF/H, and PTBP1) or modulated in opposite direction with respect to their effect on Spo11 splicing (hnRNPA1, SLM2, and ETR-3). Thus, the switch in SPO11 isoforms is unlikely due to differential expression of specific splicing factors during meiosis.
a Sequence of mouse (upper) and human (lower) exon 2 sequence showing the consensus motifs for splicing factors (SpliceAid2; http://193.206.120.249/splicing_tissue.html). Bar height represents the relative strength of the consensus. b Schematic representation of the minigene encoding the alternatively spliced region of the mouse Spo11 gene from exon 1 to exon 3, including introns. c Representative RT-PCR analysis of five experiments of HEK293T cells, untransfected, transfected with the Spo11 minigene or empty vector (pCDNA3) (left panel). The right panel shows the comparison with splicing of the endogenous Spo11 gene in P14 testis. d, e RT-PCR analysis of splicing assay performed in HEK293T cells transfected with the Spo11 minigene and expression vectors for the indicated splicing factors (d); bar graph represents densitometric analysis of the assay (e; SPO11α/SPO11β ratio; mean ± SD; n ≥ 3; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 related to mock, unpaired t test). f Western blot analysis of splicing factors in isolated germ cells at different ages. The asterisk indicates a nonspecific band. Coomassie blue staining of the gel was performed as loading control (see Supplementary Fig. 2C). The blots shown are representative of three images captured.
A reduced RNAPII elongation rate promotes splicing of SPO11α
RNAPII processivity within the transcription unit can also influence AS regulation7,8,9,22. Since RNAPII activity is modulated during male meiosis12,23, we asked whether Spo11 splicing is sensitive to changes in the RNAPII elongation rate. First, we measured RNAPII processivity as the ratio between expression of a distal vs. a proximal intron24. To avoid issues related to intron stability in steady state transcript levels, we analyzed pulse-labeled pre-mRNAs synthesized in vivo in a limited time window (Fig. 3a). Mice were injected with 5-ethynyl uridine (EU) and its accumulation in nascent RNAs was monitored by immunofluorescence analysis. We found that a 2-h time frame was sufficient to yield efficient labeling of spermatocytes with EU in vivo (Supplementary Fig. 3A). Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of EU-labeled transcripts showed that the RNAPII elongation rate within Spo11 is strongly reduced in P18 germ cells (Fig. 3b), concomitantly with exon 2 skipping and expression of SPO11α (Fig. 1a). Moreover, analysis of other proximal regions (introns 2 and 3) in Spo11 revealed that while all introns were efficiently represented in nascent transcripts at P14 (Fig. 3c), intron expression declined with distance from the transcriptional start site in P18 spermatocytes (Fig. 3d). These results suggest that the distal portion of the Spo11 pre-mRNA accumulates at a slower rate at P18, in line with a reduced elongation rate of the polymerase. Such reduction was selective for the Spo11 gene, as RNAPII processivity was unchanged within another meiotic gene (Sycp1) (Fig. 3b). Accordingly, by comparing P14 and P18 cell extracts we did not observe a general reduction in phosphorylation of RNAPII on Ser-2 (Supplementary Fig. 3B), which correlates with fast elongation rate25. However, chromatin immunoprecipitation (ChIP) experiments highlighted a specific reduction of RNAPII phosphorylation in the exon 2 region of Spo11 in P18 testis (Fig. 3e), which likely corresponds to slower rate and pausing of the polymerase, as we observed increased occupancy of total RNAPII near exon 2 (Fig. 3f) and a decline in nascent transcript accumulation downstream of it (Fig. 3d).
a Workflow used for detection of EU-labeled nascent RNAs. P14 and P18 mice were treated by intraperitoneal injection of EU (300 μg/g). EU-labeled newly synthesized RNAs were collected from testes 2 h after injection, biotinilated and captured by streptavidin magnetic beads for analysis. b qPCR analysis of nascent Spo11 and Sycp1 transcripts from P14 and P18 mouse testis. The graph represents the ratio between distal and proximal intron of EU-labeled RNA. (mean ± SD; n = 3; ∗∗P ≤ 0.01, unpaired t test). c, d qPCR analysis of different regions of the nascent Spo11 pre-mRNA corresponding in P14 (c) and P18 (d) mouse testis (mean ± SD; n = 3; ∗P ≤ 0.05, ∗∗P ≤ 0.01, ∗∗∗P ≤ 0.001 and ∗∗∗∗P ≤ 0.0001 related to int1, one-way ANOVA, Bonferroni’s multiple comparisons test). e, f ChIP assays of serine 2-phosphorylated (d) or total (e) RNAPII in the exon 2 region of Spo11 in testicular germ cells at P14 and P18. The bar graph shows qPCR signals expressed as enrichment relative to IgG (mean ± SD, n = 3; *P ≤ 0.05, unpaired t test).
To test whether a slow RNAPII influences Spo11 AS, we treated HEK293T cells with 5,6-dichloro-1-β-D-ribofuranosylbenzimidazole (DRB) to reduce Ser-2 phosphorylation (Fig. 4a) and the RNAPII elongation rate8. DRB treatment strongly promoted splicing of SPO11α from the minigene (Fig. 4a). To confirm that a slow polymerase is sufficient to induce this splicing switch, we engineered LNCaP cells to express an α-amanitin-resistant wild-type RPB1, encoding the large subunit of RNAPII, or the RPB1-R749H mutant, which reduces the elongation rate independently of phosphorylation26. The basal splicing and the response to DRB of LNCaP cells transfected with the Spo11 minigene was similar to what observed in HEK293T cells (Supplementary Fig. 3C, D). Moreover, expression of RPB1-R749H mimicked the effect of DRB and promoted SPO11α splicing (Fig. 4b). These results suggest that changes in the RNAPII elongation rate within the Spo11 locus modulate AS of exon 2 during meiosis.
a Western blot and RT-PCR analyses of a representative splicing assay performed in HEK293T cells transfected with the Spo11 minigene and treated with DRB (25 μg/ml) for 16 h. The bar graph represents densitometric analyses of the SPO11α/SPO11β ratio (mean ± SD, n = 3; *P ≤ 0.05, unpaired t test). The Western blot analysis shows the levels of serine 2-phosphorylated RNAPII in the HEK293T cells used for the assay. b Western blot and RT-PCR analysis of a representative Spo11 minigene splicing assay performed in LNCaP cells stably infected with the lentiviral vector pLV-Rbp1-WT-Amr or pLV-Rbp1-R749H-Amr and treated with 2 μg/ml of α-amanitin for 48 h, when endogenous RNAPII was inactive (mean ± SD, n = 3; ∗∗∗P ≤ 0.001, unpaired t test). The Western blot analysis shows the similar levels of expression of recombinant wild-type and mutant RNAPII in the infected LNCaP cells used for the experiment. The noninfected cells show absence of endogenous RNAPII after treatment with α-amanitin for 48 h. The blots shown are representative of three images captured (a, b).
A slow RNAPII favors recruitment of hnRNPH and skipping of exon 2 in Spo11 pre-mRNA
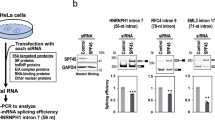
Phosphorylation of the carboxyl-terminal domain of RNAPII modulates its interaction with splicing factors during pre-mRNA processing27. As hnRNPH strongly induced SPO11α splicing, we focused on this factor. First, we evaluated whether hnRNPH interacts with RNAPII. Co-immunoprecipitation experiments revealed a weak interaction of hnRNPH with the polymerase under basal conditions (Fig. 5a). However, DRB-mediated inhibition of RNAPII Ser-2 phosphorylation promoted its association with hnRNPH (Fig. 5a), without affecting hnRNPH expression (Supplementary Fig. 4A, B). UV cross-link immunoprecipitation (CLIP) experiments demonstrated that direct binding of hnRNPH to the Spo11 pre-mRNA was also significantly enhanced by DRB treatment (Fig. 5b), suggesting that hnRNPH association with RNAPII promotes its recruitment to the target pre-mRNA. Likewise, CLIP experiments documented that hnRNPH binding to Spo11 pre-mRNA is increased in vivo in P18 testes (Fig. 5c), concomitantly with the reduction of RNAPII processivity (Fig. 3b) and SPO11α splicing (Fig. 1a).
a Co-immunoprecipitation analysis performed in HEK293T cells treated or not with DRB (25 μg/ml). Nuclear extracts were immunoprecipitated with control IgGs or anti-RNAPII antibodies in the absence of RNAse A and analyzed by Western blot for total and serine 2-phoshporylated RNAPII and hnRNPH. Nucleolin (NCL) was used as loading control for the nuclear extracts. b CLIP assay of hnRNPH binding to the Spo11 minigene in transfected HEK293T cells treated or not with DRB (25 μg/ml). Cells were UV-cross-linked and immunoprecipitated with control IgGs or anti-hnRNPH antibody. The Western blot shows specific immunoprecipitation of hnRNPH. The bar graph shows qPCR signals amplified from the CLIP assays expressed as Spo11 exon 2 enrichment in the anti-hnRNPH immunoprecipitates relative to control IgGs (mean ± SD; n = 3; ***P ≤ 0.001, unpaired t test). c CLIP assay of hnRNPH binding to the endogenous Spo11 pre-mRNA. P14 and P18 mouse testes were UV-cross-linked and immunoprecipitated with control IgGs or anti-hnRNPH antibody. The Western blot shows specific immunoprecipitation of hnRNPH. The bar graph shows qPCR signals amplified from the CLIP assays expressed as Spo11 exon 2 enrichment in the anti-hnRNPH immunoprecipitates relative to control IgGs (mean ± SD; n = 3; ***P ≤ 0.001, unpaired t test). d RT-PCR analysis of Spo11 minigene splicing assays in HEK293T cells transfected with scramble and hnRNPF/H siRNAs. Silencing efficiency was assessed by Western blot analysis. Bar graph represents the densitometric analysis of the SPO11α/SPO11β ratio (mean ± SD; n = 3; *P ≤ 0.05, ***P ≤ 0.001, unpaired t test). The blots shown are representative of three images captured (a–d).
Next, we asked if expression of hnRNPH is required for the effect of DRB on Spo11 minigene splicing. Knockdown of hnRNPH, and of its close homolog hnRNPF, reduced SPO11α splicing under basal conditions. Moreover, hnRNPF/H silencing almost completely abrogated the effect of DRB on the promotion of this splice variant (Fig. 5d), indicating that hnRNPH is involved in coupling RNAPII dynamics with Spo11 splicing regulation.
A slow RNAPII was shown to promote ETR-3 recruitment and exon skipping in the CFTR gene9. Since ETR-3 binding sites are present in the Spo11 alternatively spliced region (Fig. 2a, Supplementary Fig. 2A) and ETR-3 weakly promotes SPO11α splicing (Fig. 2d, e), we asked whether it also acted in coupling RNAPII with Spo11 AS. However, silencing of ETR-3 did not significantly modify the effect of DRB on exon 2 splicing and ETR-3 was bound to Spo11 transcript in vivo only at P14 (Supplementary Fig. S4C, D). These experiments indicate a specific role for hnRNPH in the regulation of Spo11 AS. Moreover, they suggest a combinatorial control of this event elicited by changes in RNAPII dynamics and the specific exon environment that selectively favor hnRNPH recruitment.
hnRNPH competes with Sam68 for the regulation of Spo11 AS
HnRNPs often repress splicing by competing with recruitment of other factors or the spliceosome1,4. To test whether occupancy of regulatory sequences by hnRNPH promoted exon skipping by competing out positive regulators, we focused on Sam68 because its binding site is located in close proximity to that of hnRNPH (Fig. 6a). Sam68 promotes exon 2 inclusion (Fig. 2d, e) and it cooperates with the spliceosomal U1snRNP in exon recognition28,22. Analysis of testes from juvenile Sam68 knockout mice (P10) showed a significant reduction in Spo11 exon 2 inclusion (Fig. 6b, c), suggesting the physiological relevance of Sam68-mediated regulation. To test whether hnRNPH competes with Sam68 for Spo11 AS regulation, we performed splicing assays with a concentration of Sam68 that induced almost complete splicing of the SPO11β variant and increasing amounts of hnRNPH. We observed a dose-dependent reversion of splicing with increasing hnRNPH levels (Fig. 6d, e). However, even at its highest dose, hnRNPH was unable to completely switch splicing toward the SPO11α variant in the presence of Sam68 overexpression.
a Schematic representation of the possible competition between hnRNPH and dimeric Sam68 for the binding to Spo11 exon 2 near the 5′ splice site. b Representative RT-PCR analysis of endogenous Spo11 mRNA in germ cells extracted from wild-type (wt) or Sam68 knockout (ko) testes. c Bar graph represents the densitometric analysis of the SPO11α/SPO11β ratio in germ cells extracted from wild-type (wt) or Sam68 knockout (ko) testes (mean ± SD; n = 4; *P ≤ 0.05, unpaired t test). d RT-PCR analysis of Spo11 minigene splicing assay performed in HEK293T cells transfected with or without Flag-Sam68 or increasing amounts of Flag-hnRNPH. Relative expression of Sam68 and hnRNPH was detected by Western blot using the anti-FLAG antibody. e Bar graph represents densitometric analysis of the SPO11α/SPO11β ratio of Spo11 minigene splicing assay performed in HEK293T cells transfected with or without Flag-Sam68 or increasing amounts of Flag-hnRNPH (mean ± SD; n = 3, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, unpaired t test). f CLIP assays of MYC-Sam68 binding to the Spo11 pre-mRNA. HEK293T cells were transfected with the Spo11 minigene and Myc-Sam68 or Flag-hnRNPH. Immunoprecipitation of Myc-Sam68 was analyzed by Western blot with anti-MYC antibody. The bar graph shows qPCR signals of Spo11 exon 2 amplified from the CLIP assays expressed as enrichment in the anti-MYC-Sam68 immunoprecipitates in Myc-Sam68 expressing cells relative to cells expressing the MYC empty vector (mean ± SD; n = 3; **p ≤ 0.01, unpaired t test). g CLIP assay of endogenous Sam68 binding to the Spo11 minigene in HEK293T cells transfected with scramble and hnRNPF/H siRNAs. Silencing efficiency was assessed by Western blot analysis (Supplementary Fig. S5). Cells were immunoprecipitated with control IgGs or anti-Sam68 antibody. The Western blot shows specific immunoprecipitation of Sam68. The bar graph shows qPCR signals amplified from the CLIP assays expressed as Spo11 exon 2 enrichment in the anti-Sam68 immunoprecipitates relative to control IgGs (mean ± SD; n = 3; *P ≤ 0.05, unpaired t test). The blots shown are representative of three images captured (d, f, g).
To test whether hnRNPH competes with Sam68 for binding to exon 2, we performed CLIP assays in HEK293T cells transfected with the Spo11 minigene. Sam68 was efficiently recruited to the Spo11 pre-mRNA when expressed alone. However, its binding was strongly reduced when hnRNPH was overexpressed (Fig. 6f). By contrast, hnRNPF/H knockdown caused a small but significant increase in the binding of Sam68 to the Spo11 pre-mRNA (Fig. 6g). These findings suggest that binding of hnRNPH near specific regulatory regions hinders the recruitment of Sam68, thus promoting exon 2 skipping from the Spo11 pre-mRNA.
Discussion
Male germ cells display the highest complexity in gene expression and AS regulation among mammalian tissues10,12. This extensive utilization of the coding potential and plasticity of the genome may underlie the unique features required for germ cell differentiation29. One such unique feature is the timely appearance in early meiosis of DSBs driven by SPO1115,16, which allow recombination, pairing and proper segregation of homologous chromosomes30. Notably, the main SPO11 splice variants, SPO11α and β, are differentially expressed in spermatocytes and both are necessary for proper meiosis18. Nevertheless, how temporal regulation of Spo11 splicing is achieved is currently unknown. Herein, we have identified a combinatorial control that insures Spo11 AS regulation at a specific time in meiosis, which relies on the concerted action of RNAPII and the splicing factor hnRNPH. A fast RNAPII elongation rate in early meiosis prevents binding of hnRNPH to the Spo11 pre-mRNA, whereas the reduced speed of RNAPII in late meiosis promotes hnRNPH recruitment and skipping of exon 2 (Fig. 7). These findings uncover a novel modality of developmental regulation of splicing that couples changes in RNAPII dynamics with specific recruitment of splicing factors during meiosis, thus guaranteeing accurate control of SPO11 isoform expression in the differentiating gametes.
Schematic representation of the combinatorial control of Spo11 alternative splicing elicited by RNAPII dynamics and hnRNPH or Sam68 recruitment. At P14, the fast elongation rate of RNAPII promotes recruitment of Sam68 and inclusion of exon 2, thus yielding SPO11β. At P18, reduction of the speed of RNAPII favors recruitment of hnRNPH, which competes out Sam68 and promotes exon 2 skipping and SPO11α splicing.
HnRNPH, and its close homolog hnRNPF, are potent regulators of exon 2 skipping. Interestingly, in primary germ cells hnRNPH is differentially recruited in proximity of the Spo11 exon 2 splice site at P14 and P18, even though its expression level remains unchanged. However, we found that recruitment of hnRNPH to Spo11 exon 2 sequences is sensitive to RNAPII dynamics, whose regulation during meiosis has been documented12,31,32. Several lines of evidence support this conclusion. First, interaction of hnRNPH and RNAPII is promoted by Ser-2 dephosphorylation; second, RNAPII phosphorylation in Ser-2 and RNAPII processivity within the Spo11 locus are modulated during meiosis; third, decreased processivity correlates with recruitment of hnRNPH to exon 2 in the Spo11 pre-mRNA; fourth, ectopic manipulation of RNAPII dynamics recapitulates regulation of SPO11α splicing in an hnRNPH-dependent fashion. These findings suggest that a fast RNAPII elongation rate prevents binding of hnRNPF/H to the Spo11 pre-mRNA, allowing its recognition by positive regulators that promote SPO11β splicing (Fig. 7). Reduced speed of RNAPII later in meiosis, instead, favors recruitment of hnRNPH, leading to displacement of positive regulators and splicing of SPO11α. This competition-based mechanism is also supported by the observation that the strongest hnRNPH binding sites in exon 2 overlap with the consensus motif for Sam68, which promotes SPO11β splicing. Thus, occupancy of the region flanking the 5′ splice site by hnRNPH may prevent recruitment of positive regulators (i.e., Sam68) that are necessary for efficient exon 2 definition. Mechanistically, since Sam68 cooperates with U1snRNP to promote exon recognition in male germ cells31, binding of hnRNPH to the exon 2–intron 2 region may disrupt this interaction at P18 and cause the switch in SPO11 isoforms. Thus, our work suggests that combinatorial control of RNAPII dynamics and recruitment of specific splicing factors may dictate temporal regulation of a splicing pattern during meiosis.
The physiological relevance of most annotated splice variants remains largely unknown. In the case of SPO11, expression of SPO11β alone was not sufficient to fully compensate for the ablation of the gene18. SPO11β-only mice were defective in pairing of the X–Y chromosomes in late meiosis, suggesting that SPO11α plays a nonredundant role and physiologically compensates for this defect18. This observation also indicates that lack of proper SPO11α splicing during meiosis exposes to higher risk of sex chromosomes aneuploidy, a pathological condition that gives rise to human diseases like the Klinefelter syndrome33. Thus, while changes in Spo11 expression levels can be tolerated to some extent by homeostatic mechanisms that control crossover formation34, lack of expression of a splice variant of the gene yields non rescuable defects. In this scenario, our findings provide mechanistic insights into the temporal control of Spo11 splicing during meiosis and point to hnRNPH and Sam68 as key regulators of this process. Notably, Sam68 knockout spermatocytes undergo apoptosis in late meiosis19. Thus, our findings also suggest that dysregulation of SPO11α expression may cause defective homologous recombination in vivo, resulting in increased meiotic cell death.
Materials and methods
Germ cells isolation and culture
Germ cells were obtained from testes of Swiss CD-1 mice as reported23. Briefly, after testis digestion with collagenase and trypsin, the cell suspension was plated for 4 h in minimum essential medium (MEM), supplemented with 1 mM dl-lactic acid, 2 mM sodium pyruvate, 10% fetal calf serum, to promote adhesion of somatic cells. Germ cells that remain in suspension were then collected. For transcription inhibition, following two washes in MEM, germ cells were released for 6 h in fresh medium added or not with 1 μM FPD (Sigma-Aldrich) and then harvested for analysis.
Spo11 minigene construct and PCR analyses
The Spo11 minigene was amplified from genomic DNA of P14 mouse testis using primers reported in the Supplementary Table, cloned into pCDNA3.1(−) vector, and validated by sequencing.
For PCR analyses, RNA from samples was extracted using TRIzol reagent (Invitrogen), digested with RNase-free DNase (Roche), retrotranscribed (1 μg) using M-MLV reverse transcriptase (Promega) and used for PCR reactions (GoTaq, Promega). RT-qPCR analyses were performed using LightCycler 480 SYBR Green I Master with the LightCycler 480 System (Roche). Control reactions omitted M-MLV reverse transcriptase. Primers are listed in the Supplementary Table 1.
Cell culture, transfections, and treatment
HEK293T cells were grown in Dulbecco’s modified Eagle’s medium (Sigma Aldrich), supplemented with 10% fetal bovine serum (FBS) (Gibco), gentamicin sulfate (50 g/ml) (Aurogene), 1% nonessential aminoacids (Euroclone), penicillin (50 U/ml)/streptomycin (50 g/ml) (Corning). LNCaP cells were grown in RPMI 1640 medium (LONZA), supplemented with 10% FBS (Gibco), penicillin (50 U/ml)/streptomycin (50 g/ml) (Corning), gentamicin sulfate (50 g/ml) (Aurogene), 1% nonessential aminoacids (Euroclone), 10 mM Hepes (Euroclone), and 1 mM sodium pyruvate (Aurogene). Transfections were performed using Lipofectamine 2000 (Invitrogen) and 2 μg of pCDNA3.1-Spo11 minigene with or without expression vectors for the indicated proteins; 6 h after transfection, cells were treated or not with 25 μg/ml DRB for 16 h before harvest. For RNA interference, cells were transfected with appropriate siRNAs (Sigma-Aldrich) using Lipofectamine RNAiMAX (Invitrogen) and harvested 48 h later for analyses.
Pull-down assay of nascent RNAs
Testis of P14 and P18 mice were collected after 2 h of intraperitoneal injection of EU (Life Technologies) or PBS as control, as described12. Samples were collected in Trizol and RNA was isolated, biotinylated and captured using the Click-IT Nascent RNA Capture kit (Life Technologies). Captured nascent RNAs were retrotranscribed using the SuperScript VILO cDNA Synthesis Kit (Life Technologies) followed by qPCR analysis.
Cross-link immunoprecipitation (CLIP) experiments
CLIP experiments were performed as described35. Briefly, HEK293T cells and testicular cells were irradiated on ice (400 mJ/cm2) in PBS, scraped off and centrifuged for 5 min at 300g at 4 °C. After sample processing, extracts (0.5–1 mg) were immunoprecipitated using anti-hnRNPH (provided by Prof. B. Chabot, Université de Sherbrooke, Canada), anti-MYC (Santa Cruz Biotechnology) or rabbit IgG (control) in the presence of protein G magnetic (Life Technologies). After stringent washes and Proteinase K treatment (1 h at 55 °C), RNA was isolated by standard procedures, retrotranscribed with random primers, and M-MLV reverse transcriptase (Promega) and used for qPCR. RNA associated with hnRNPH is represented as fold enrichment relative to IgG samples, RNA associated with MYC is represented as fold enrichment relative to empty vector.
ChIP assay
ChIP was performed as described36 using P14 and P18 testes crosslinked in 1% (vol/vol) formaldehyde for 10 min at room temperature and quenched with 125 mM glycine for 5 min.
Statistical analysis
Statistical tests were performed by the Student t test or One-Way ANOVA using GraphPad Prism6; P ≤ 0.05 was considered significant. No analyses were used to predetermine sample size. The experiments were not randomized and investigators were not blinded to allocation during experiments and outcome assessment.
References
Fu, X. & Ares, M. J. Context-dependent control of alternative splicing by RNA binding proteins. Nat. Rev. Genet. 15, 689–701 (2014).
Paronetto, M. P., Passacantilli, I. & Sette, C. Alternative splicing and cell survival: from tissue homeostasis to disease. Cell Death Differ. 23, 1919–1929 (2016).
Will, C. L. & Lührmann, R. Spliceosome structure and function. Cold Spring Harb. Perspect. Biol. 3, 1–2 (2011).
Matera, A. G. & Wang, Z. A day in the life of the spliceosome. Nat. Rev. Mol. Cell Biol. 15, 108–121 (2014).
Luco, R. F., Allo, M., Schor, I. E., Kornblihtt, A. R. & Misteli, T. Epigenetics in alternative pre-mRNA splicing. Cell 144, 16–26 (2011).
Naftelberg, S., Schor, I. E., Ast, G. & Kornblihtt, A. R. Regulation of alternative splicing through coupling with transcription and chromatin structure. Annu. Rev. Biochem. 84, 165–198 (2015).
De La Mata, M. et al. A slow RNA polymerase II affects alternative splicing in vivo. Mol. Cell 12, 525–532 (2003).
Ip, J., Schmidt, D. & Pan, Q. Global impact of RNA polymerase II elongation inhibition on alternative splicing regulation. Genome Res. 21, 390–401 (2011).
Dujardin, G. et al. How slow RNA polymerase II elongation favors alternative exon skipping. Mol. Cell 54, 683–690 (2014).
Soumillon, M. et al. Cellular source and mechanisms of high transcriptome complexity in the mammalian testis. Cell Rep. 3, 2179–2190 (2013).
Schmid, R. et al. The splicing landscape is globally reprogrammed during male meiosis. Nucleic Acids Res. 41, 10170–10184 (2013).
Naro, C. et al. An orchestrated intron retention program in meiosis controls timely usage of transcripts during germ cell differentiation. Dev. Cell 41, 82–93.e4 (2017).
Elliott, D. J. & Grellscheid, S. N. Alternative RNA splicing regulation in the testis. Reproduction 132, 811–819 (2006).
Paronetto, M. P. & Sette, C. Role of RNA-binding proteins in mammalian spermatogenesis. Int J. Androl. 33, 2–12 (2010).
Baudat, F., Manova, K., Yuen, J. P., Jasin, M. & Keeney, S. Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol. Cell 6, 989–998 (2000).
Romanienko, P. J. & Camerini-Otero, R. D. The mouse Spo11 gene is required for meiotic chromosome synapsis. Mol. Cell 6, 975–987 (2000).
Bellani, M. A., Boateng, K. A., McLeod, D. & Camerini-Otero, R. D. The expression profile of the major mouse SPO11 isoforms indicates that SPO11 introduces double strand breaks and suggests that SPO11 has an additional role in prophase in both spermatocytes and oocytes. Mol. Cell Biol. 30, 4391–4403 (2010).
Kauppi, L. et al. Distinct properties of the XY pseudoautosomal region crucial for male meiosis. Science 331, 916–920 (2011).
Paronetto, M. P. et al. Sam68 regulates translation of target mRNAs in male germ cells, necessary for mouse spermatogenesis. J. Cell Biol. 185, 235–249 (2009).
Bellve, A. R. et al. Spermatogenic cells of the prepubertal mouse. Isolation and morphological characterization. J. Cell Biol. 74, 68–85 (1977).
Moens, P. B., Heyting, C., Dietrich, A. J., vanRaamsdonk, W. & Chen, Q. Synaptonemal complex antigen localization and conservation. J. Cell Biol. 105, 93–103 (1987).
Subramania, S. et al. SAM68 interaction with U1A modulates U1 snRNP recruitment and regulates mTor pre-mRNA splicing. Nucleic Acids Res. 47, 4181–4197 (2019).
Paronetto, M. P. et al. Sam68 marks the transcriptionally active stages of spermatogenesis and modulates alternative splicing in male germ cells. Nucleic Acids Res. 39, 4961–4974 (2011).
Batsché, E., Yaniv, M. & Muchardt, C. The human SWI/SNF subunit Brm is a regulator of alternative splicing. Nat. Struct. Mol. Biol. 13, 22–29 (2006).
Phatnani, H. P. & Greenleaf, A. L. Phosphorylation and functions of the RNA polymerase IICTD. Genes Dev. 20, 2922–2936 (2006).
Fong, N. et al. Pre-mRNA splicing is facilitated by an optimal RNA polymerase II elongation rate. Genes Dev. 28, 2663–2676 (2014).
Hsin, J. & Manley, J. L. The RNA polymerase II CTD coordinates transcription and RNA processing. Genes Dev. 26, 2119–2137 (2012).
Naro, C. et al. Functional Interaction between U1snRNP and Sam68 insures proper 3′ end pre-mRNA processing during germ cell differentiation. Cell Rep. 26, 2929–2941.e5 (2019).
Griswold, M. D. Spermatogenesis: the commitment to meiosis. Physiol. Rev. 96, 1–17 (2016).
Keeney, S., Lange, J. & Mohibullah, N. Self-organization of meiotic recombination initiation: general principles and molecular pathways. Annu. Rev. Genet. 48, 187–214 (2014).
Turner, J. M. A. et al. Silencing of unsynapsed meiotic chromosomes in the mouse. Nat. Genet. 37, 41–47 (2005).
Bielli, P., Busà, R., Paronetto, M. P. & Sette, C. The RNA-binding protein Sam68 is a multifunctional player in human cancer. Endocr. Relat. Cancer 18, R91–R102 (2011).
Thomas, N. S. & Hassold, T. J. Aberrant recombination and the origin of Klinefelter syndrome. Hum. Reprod. Update 9, 309–317 (2003).
Cole, F. et al. Homeostatic control of recombination is implemented progressively in mouse meiosis. Nat. Cell Biol. 14, 424–430 (2012).
Bielli, P. & Sette, C. Analysis of in vivo interaction between RNA binding proteins and their RNA targets by UV cross-linking and immunoprecipitation (CLIP) method. Bio-Protocol. https://doi.org/10.21769/bioprotoc.2274 (2017).
Cappellari, M. et al. The transcriptional co-activator SND1 is a novel regulator of alternative splicing in prostate cancer cells. Oncogene 33, 3794–3802 (2014).
Acknowledgements
We wish to thank Dr. M. Barchi for fruitful discussion throughout this work and Drs. M.A. Handel, B. Chabot, D. Elliott for providing antibodies used in this study, Dr. E. Buratti for providing the TDP-43 vector. This work was supported by Telethon (GGP12189), by Associazione Italiana Ricerca sul Cancro (AIRC) and by the UCSC grant Linea D1. C.N. was supported in part by a scholarship from Fondazione Umberto Veronesi. Supplementary information is available at Cell Death & Disease’s website.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Statements: We declare that our work is original and lack any form of plagiarism and image manipulation to alter the significance of the data.
Edited by G. Melino
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cesari, E., Loiarro, M., Naro, C. et al. Combinatorial control of Spo11 alternative splicing by modulation of RNA polymerase II dynamics and splicing factor recruitment during meiosis. Cell Death Dis 11, 240 (2020). https://doi.org/10.1038/s41419-020-2443-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-020-2443-y
This article is cited by
-
High-throughput RNA sequencing reveals differences between the transcriptomes of the five spore forms of Puccinia striiformis f. sp. tritici, the wheat stripe rust pathogen
Stress Biology (2023)
-
hnRNPH1 recruits PTBP2 and SRSF3 to modulate alternative splicing in germ cells
Nature Communications (2022)