Abstract
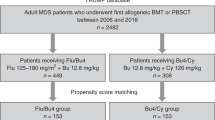
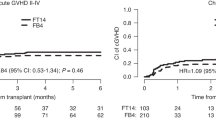
Fludarabine and a myeloablative dose of busulfan (Flu/Bu4) can improve prognosis after allogeneic hematopoietic stem cell transplantation (HSCT) with melphalan (Mel). We investigated the prognostic impact of adding Mel to Flu/Bu4 by comparing between Flu/Bu4/Mel and Flu/Bu4 groups. This study included 846 propensity score (PS)-matched patients who received either Flu/Bu4/Mel (n = 423) or Flu/Bu4 (n = 423) from 2394 patients enrolled in a multicenter prospective registry, from January 2010 to December 2016. The primary endpoint (5-year overall survival [OS]), and the prognostic impact of adding Mel was evaluated using Cox regression analysis. The study population median age was 58 (interquartile 50–64) years and 61.0% were male. Patient characteristics were well-balanced between groups. Five-year OS was 34.2% (95% confidence interval [CI]: 27.3–41.1%) and 30.1% (24.8–35.6%) in the Flu/Bu4/Mel and Flu/Bu4 groups, respectively (log-rank P = 0.019). The adjusted hazard ratio of adding Mel was 0.77 (95% CI: 0.62–0.96) (P = 0.022) for the 5-year OS, and this attributed to a lower incidence of 5-year relapse (0.71, 0.56–0.90, P = 0.005) and relapse associated mortality (0.73, 0.57–0.95, P = 0.018). There was no statistical difference in 5-year non-relapse mortality between groups (log-rank P = 0.855). Flu/Bu4/Mel was associated with better 5-year OS compared to Flu/Bu4 in a PS-matched cohort after allogeneic HSCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol J Am Soc Clin Oncol. 2010;28:3730–8.
Le Bourgeois A, Labopin M, Blaise D, Ceballos P, Vigouroux S, Peffault De Latour R. et al. Reduced-intensity versus reduced-toxicity myeloablative fludarabine/busulfan-based conditioning regimens for allografted non-Hodgkin lymphoma adult patients: a retrospective study on behalf of the Société Francophone de Greffe de Moelle et de Thérapie Cellulaire. Ann Oncol J Eur Soc Med Oncol. 2017;28:2191–8.
Russell JA, Tran HT, Quinlan D, Chaudhry A, Duggan P, Brown C, et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2002;8:468–76.
de Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–64.
Ben-Barouch S, Cohen O, Vidal L, Avivi I, Ram R. Busulfan fludarabine vs busulfan cyclophosphamide as a preparative regimen before allogeneic hematopoietic cell transplantation: systematic review and meta-analysis. Bone Marrow Transpl. 2016;51:232–40.
Lee J-H, Joo Y-D, Kim H, Ryoo HM, Kim MK, Lee GW, et al. Randomized trial of myeloablative conditioning regimens: busulfan plus cyclophosphamide versus busulfan plus fludarabine. J Clin Oncol J Am Soc Clin Oncol. 2013;31:701–9.
Speziali C, Daly A, Abuhaleeqa M, Nitta J, Abou Mourad Y, Seftel MD, et al. Fludarabine, busulfan, and low-dose TBI conditioning versus cyclophosphamide and TBI in allogeneic hematopoietic cell transplantation for adult acute lymphoblastic leukemia. Leuk Lymphoma. 2019;60:639–48.
Gyurkocza B, Lazarus HM, Giralt S. Allogeneic hematopoietic cell transplantation in patients with AML not achieving remission: potentially curative therapy. Bone Marrow Transpl. 2017;52:1083–90.
Bayraktar UD, Bashir Q, Qazilbash M, Champlin RE, Ciurea SO. Fifty years of melphalan use in hematopoietic stem cell transplantation. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2013;19:344–56.
Kebriaei P, Madden T, Wang X, Thall PF, Ledesma C, De Lima M, et al. Intravenous BU plus Mel: an effective, chemotherapy-only transplant conditioning regimen in patients with ALL. Bone Marrow Transpl. 2013;48:26–31.
Small TN, Young JW, Castro-Malaspina H, Prockop S, Wilton A, Heller G, et al. Intravenous busulfan and melphalan, tacrolimus, and short-course methotrexate followed by unmodified HLA-matched related or unrelated hematopoietic stem cell transplantation for the treatment of advanced hematologic malignancies. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2007;13:235–44.
Yamamoto H, Uchida N, Yuasa M, Kageyama K, Ota H, Kaji D, et al. A novel reduced-toxicity myeloablative conditioning regimen using full-dose busulfan, fludarabine, and melphalan for single cord blood transplantation provides durable engraftment and remission in nonremission myeloid malignancies. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2016;22:1844–50.
Ueda T, Maeda T, Kusakabe S, Fujita J, Fukushima K, Yokota T, et al. Addition of melphalan to fludarabine/busulfan (FLU/BU4/MEL) provides survival benefit for patients with myeloid malignancy following allogeneic bone-marrow transplantation/peripheral blood stem-cell transplantation. Int J Hematol 2019;109:197–205.
Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013;32:2837–49.
Yamamoto H, Uchida N, Matsuno N, Kon A, Nishida A, Ota H, et al. I.v. BU/fludarabine plus melphalan or TBI in unrelated cord blood transplantation for high-risk hematological diseases. Bone Marrow Transpl. 2015;50:607–9.
Horwitz ME, Morris A, Gasparetto C, Sullivan K, Long G, Chute J, et al. Myeloablative intravenous busulfan/fludarabine conditioning does not facilitate reliable engraftment of dual umbilical cord blood grafts in adult recipients. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2008;14:591–4.
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–90.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Arai Y, Takeda J, Aoki K, Kondo T, Takahashi S, Onishi Y, et al. Efficiency of high-dose cytarabine added to CY/TBI in cord blood transplantation for myeloid malignancy. Blood. 2015;126:415–22.
Davies SM, Kollman C, Anasetti C, Antin JH, Gajewski J, Casper JT, et al. Engraftment and survival after unrelated-donor bone marrow transplantation: a report from the national marrow donor program. Blood. 2000;96:4096–102.
Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344:175–81.
Akpek G, Pasquini MC, Logan B, Agovi M-A, Lazarus HM, Marks DI, et al. Effects of spleen status on early outcomes after hematopoietic cell transplantation. Bone Marrow Transpl. 2013;48:825–31.
Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–9.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transpl. 1995;15:825–8.
Sora F, Grazia CD, Chiusolo P, Raiola AM, Bregante S, Mordini N, et al. Allogeneic hemopoietic stem cell transplants in patients with acute myeloid leukemia (AML) prepared with busulfan and fludarabine (BUFLU) or thiotepa, busulfan, and fludarabine (TBF): a retrospective study. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2020;26:698–703.
Rambaldi A, Grassi A, Masciulli A, Boschini C, Micò MC, Busca A, et al. Busulfan plus cyclophosphamide versus busulfan plus fludarabine as a preparative regimen for allogeneic haemopoietic stem-cell transplantation in patients with acute myeloid leukaemia: an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2015;16:1525–36.
Saraceni F, Labopin M, Hamladji R-M, Mufti G, Socié G, Shimoni A, et al. Thiotepa-busulfan-fludarabine compared to busulfan-fludarabine for sibling and unrelated donor transplant in acute myeloid leukemia in first remission. Oncotarget. 2018;9:3379–93.
Sanz J, Boluda JCH, Martín C, González M, Ferra C, Serrano D, et al. Single-unit umbilical cord blood transplantation from unrelated donors in patients with hematological malignancy using busulfan, thiotepa, fludarabine and ATG as myeloablative conditioning regimen. Bone Marrow Transpl. 2012;47:1287–93.
Mehta RS, Di Stasi A, Andersson BS, Nieto Y, Jones R, De Lima M, et al. The development of a myeloablative, reduced-toxicity, conditioning regimen for cord blood transplantation. Clin Lymphoma Myeloma Leuk. 2014;14:e1–5.
Ringdén O, Labopin M, Ehninger G, Niederwieser D, Olsson R, Basara N, et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol J Am Soc Clin Oncol 2009;27:4570–7.
Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes. J Clin Oncol J Am Soc Clin Oncol 2017;35:1154–61.
Kröger N, Iacobelli S, Franke G-N, Platzbecker U, Uddin R, Hübel K, et al. Dose-reduced versus standard conditioning followed by allogeneic stem-cell transplantation for patients with myelodysplastic syndrome: a prospective randomized phase III study of the EBMT (RICMAC Trial). J Clin Oncol J Am Soc Clin Oncol. 2017;35:2157–64.
Ciurea SO, Andersson BS. Busulfan in hematopoietic stem cell transplantation. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2009;15:523–36.
Li L, Liu X, Glassman AB, Keating MJ, Stros M, Plunkett W, et al. Fludarabine triphosphate inhibits nucleotide excision repair of cisplatin-induced DNA adducts in vitro. Cancer Res. 1997;57:1487–94.
Gandhi V, Plunkett W. Cellular and clinical pharmacology of fludarabine. Clin Pharmacokinet. 2002;41:93–103.
Bacigalupo A, Van Lint MT, Occhini D, Gualandi F, Lamparelli T, Sogno G, et al. Increased risk of leukemia relapse with high-dose cyclosporine A after allogeneic marrow transplantation for acute leukemia. Blood. 1991;77:1423–8.
Baron F, Labopin M, Niederwieser D, Vigouroux S, Cornelissen JJ, Malm C, et al. Impact of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation for acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European group for blood and marrow transplantation. Leukemia. 2012;26:2462–8.
Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2009;15:367–9.
Acknowledgements
The authors thank all the physicians and data managers of the centers, who contributed to the collection of data on transplantation to the Japanese Data Center for Hematopoietic Cell Transplantation and the Transplant Registry Unified Management Program 2. We would like to express our gratitude to the Japan Society of Clinical Research for their dedicated support. We presented a part of this study at the 2019 American Society of Hematology Annual Meeting (Orlando, FL, December 7-10, 2019)
Author information
Authors and Affiliations
Contributions
YS designed the study, analyzed the data, performed the statistical analysis, and wrote the first draft of the paper. MH, HY, and TI contributed a critical review of the analysis data, interpretation of the data, and the paper. All the other authors contributed to data collection and critical review of the paper. All authors approved the final version and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shimomura, Y., Hara, M., Yamamoto, H. et al. Adding melphalan to fludarabine and a myeloablative dose of busulfan improved survival after allogeneic hematopoietic stem cell transplantation in a propensity score-matched cohort of hematological malignancies. Bone Marrow Transplant 56, 1691–1699 (2021). https://doi.org/10.1038/s41409-021-01217-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-021-01217-w
This article is cited by
-
Updated comparable efficacy of cord blood transplantation for chronic myelomonocytic leukaemia: a nationwide study
Bone Marrow Transplantation (2024)
-
Comparison of fludarabine, a myeloablative dose of busulfan, and melphalan vs conventional myeloablative conditioning regimen in patients with relapse and refractory acute myeloid leukemia in non-remission status
Bone Marrow Transplantation (2021)