Abstract
Background
Metformin may have anticancer effects that are independent of its hypoglycemic effects. Retrospective studies have shown that metformin use is associated with decreased incidence of prostate cancer and prostate cancer-specific mortality. Preclinical studies suggesting additive anticancer effects of combining metformin and bicalutamide prompted this clinical trial (NCT02614859).
Methods
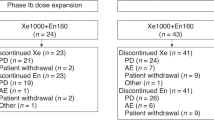
This open-label, randomized, phase 2 trial enrolled non-diabetic patients with biochemically recurrent prostate cancer, a PSADT of 3–9 months, BMI > 25 and normal testosterone. Patients were randomized 1:2 to observation for an initial 8 weeks (Arm A) or metformin 1000 mg twice daily (Arm B). Bicalutamide 50 mg/day was added after 8 weeks to both arms. The primary objective was to evaluate the number of patients with undetectable PSA ( < 0.2 ng/mL) at the end of 32 weeks. Immune correlatives were assessed as exploratory endpoints.
Results
A total of 29 patients were enrolled from March 2015 to January 2020. No difference was seen between the 2 arms in the proportion of patients with undetectable PSA. Modest PSA decrease ranging from 4% to 24% were seen in 40.0% (95% CI: 19.1–64.0%) of patients with metformin monotherapy, compared to 11.1% (95% CI: 0.3–48.3%) in the observation arm. Metformin monotherapy reduced PD-1+ NK cells, and increased NKG2D+ NK cells. The combination of metformin and bicalutamide led to greater reductions in PD-1 expressing NK, CD4+ T, and CD8+ T-cell subsets compared to bicalutamide alone. The trial was stopped early due to predicted inability to achieve its primary endpoint.
Conclusions
Although metformin plus bicalutamide was well tolerated, there was no improvement in rates of achieving undetectable PSA at 32 weeks. Metformin monotherapy induced modest PSA declines in 40% of patients after 8 weeks. Metformin, given alone and in combination with bicalutamide, displayed immune modifying effects, primarily within NK and T cells subsets.
Trial registration
Trial Registration Number: NCT02614859.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ.2005;330:1304–5.
Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care. 2010;33:322–6.
Frasca F, Pandini G, Sciacca L, Pezzino V, Squatrito S, Belfiore A, et al. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem. 2008;114:23–37.
Mistry T, Digby JE, Desai KM, Randeva HS. Obesity and prostate cancer: a role for adipokines. Eur Urol. 2007;52:46–53.
Hsing AW, Gao YT, Chua S Jr., Deng J, Stanczyk FZ. Insulin resistance and prostate cancer risk. J Natl Cancer Inst. 2003;95:67–71.
Freedland SJ, Aronson WJ, Kane CJ, Presti JC Jr., Amling CL, Elashoff D, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004;22:446–53.
Cao Y, Ma J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Philos). 2011;4:486–501.
Rothermundt C, Hayoz S, Templeton AJ, Winterhalder R, Strebel RT, Bartschi D, et al. Metformin in chemotherapy-naive castration-resistant prostate cancer: a multicenter phase 2 trial (SAKK 08/09). Eur Urol. 2014;66:468–74.
Joshua A, Zannella V, Bowes B, Koritzinsky M, Sweet J, Evans A, et al. A phase II study of neoadjuvant metformin in prostatic carcinoma. AACR Cancer Res. 2012;72:abstr CT–04.
Colquhoun AJ, Venier NA, Vandersluis AD, Besla R, Sugar LM, Kiss A, et al. Metformin enhances the antiproliferative and apoptotic effect of bicalutamide in prostate cancer. Prostate Cancer Prostatic Dis. 2012;15:346–52.
Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA.1999;281:1591–7.
Donahue RN, Marte JL, Goswami M, Toney NJ, Tsai YT, Gulley JL, et al. Interrogation of the cellular immunome of cancer patients with regard to the COVID-19 pandemic. J Immunother Cancer. 2021;9:e002087.
Lepone LM, Donahue RN, Grenga I, Metenou S, Richards J, Heery CR, et al. Analyses of 123 peripheral human immune cell subsets: defining differences with age and between healthy donors and cancer patients not detected in analysis of standard immune cell types. J Circ Biomark. 2016;5:5.
Donahue RN, Lepone LM, Grenga I, Jochems C, Fantini M, Madan RA, et al. Analyses of the peripheral immunome following multiple administrations of avelumab, a human IgG1 anti-PD-L1 monoclonal antibody. J Immunother Cancer. 2017;5:20.
Litwin S, Basickes S, Ross EA. Two-sample binary phase 2 trials with low type I error and low sample size. Stat Med. 2017;36:1383–94.
El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–8.
Dowling RJ, Niraula S, Stambolic V, Goodwin PJ. Metformin in cancer: translational challenges. J Mol Endocrinol. 2012;48:R31–43.
Cerezo M, Tichet M, Abbe P, Ohanna M, Lehraiki A, Rouaud F, et al. Metformin blocks melanoma invasion and metastasis development in AMPK/p53-dependent manner. Mol Cancer Ther. 2013;12:1605–15.
Eikawa S, Nishida M, Mizukami S, Yamazaki C, Nakayama E, Udono H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc Natl Acad Sci USA. 2015;112:1809–14.
Hirsch HA, Iliopoulos D, Struhl K. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc Natl Acad Sci USA. 2013;110:972–7.
Collette L. Prostate-specific antigen (PSA) as a surrogate end point for survival in prostate cancer clinical trials. Eur Urol. 2008;53:6–9.
Mark M, Klingbiel D, Mey U, Winterhalder R, Rothermundt C, Gillessen S, et al. Impact of addition of metformin to abiraterone in metastatic castration-resistant prostate cancer patients with disease progressing while receiving abiraterone treatment (MetAb-Pro): phase 2 pilot study. Clin Genitourin Cancer. 2019;17:e323–e28.
Parikh M, Robles D, Pan C, Lara P, Yang J, Gao A, et al. Results from a phase Ib/II study of enzalutamide and metformin in men with castration resistant prostate cancer (CRPC). J Clin Oncol. 2019;37:abstr 5054.
Martin M, Borchiellini D, Viotti J, Guillot A, Paoli J, Besson D, et al. TAXOMET: A French prospective multicenter randomized controlled phase II study comparing docetaxel plus metformin versus docetaxel plus placebo in mCRPC. J Clin Oncol. 2019;37:abstr 5004.
Chen S, Wainwright DA, Wu JD, Wan Y, Matei DE, Zhang Y, et al. CD73: an emerging checkpoint for cancer immunotherapy. Immunotherapy.2019;11:983–97.
Ma R, Yi B, Riker A, Xi Y. Metformin and cancer immunity. Acta Pharmalogica Sin. 2020;41:1403–09.
Acknowledgements
We thank the patients and associated study staff for their participation in this trial. The authors also thank Bonnie L. Casey for editorial assistance in the preparation of this manuscript. The authors additionally thank Ariana Sabzevari, Angie Schwab, and Keanan Wright for technical assistance with immune assays, and Dr. Samuel Litwin for his assistance with the statistical design for the trial.
Funding
This work was supported in part by a grant to the Fox Chase Cancer Center (#P30CA006927) from the National Cancer Institute, National Institutes of Health, and the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Conception/Design: MB, ERP, DMG. Provision of study material or patients: MZ, PG, RAM, FK, WLD, JLG, MB. Collection and/or assembly of data: SW, RND, NJT, JS, MB, DMG. Data analysis and interpretation: RND, NJT, JS, JLG, MB, DMG, ER. Manuscript writing: MB, RND, NJT, DMG. Final approval of manuscript: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Boards of the Fox Chase Cancer Center, Temple University, and the Center for Cancer Research, National Cancer Institute. The study was conducted according to the principles of the Declaration of Helsinki and was performed in compliance with Good Clinical Practice guidelines. Written informed consent was obtained from each patient.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bilusic, M., Toney, N.J., Donahue, R.N. et al. A randomized phase 2 study of bicalutamide with or without metformin for biochemical recurrence in overweight or obese prostate cancer patients (BIMET-1). Prostate Cancer Prostatic Dis 25, 735–740 (2022). https://doi.org/10.1038/s41391-022-00492-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-022-00492-y
This article is cited by
-
Metformin and cancer hallmarks: shedding new lights on therapeutic repurposing
Journal of Translational Medicine (2023)
-
Current status and frontier tracking of clinical trials on Metformin for cancer treatment
Journal of Cancer Research and Clinical Oncology (2023)
-
The promising therapeutic effects of metformin on metabolic reprogramming of cancer-associated fibroblasts in solid tumors
Cellular & Molecular Biology Letters (2022)