Abstract
Background
Tacrolimus ointment is a recently developed topical immunomodulator that has been approved for use in patients with vitiligo older than 2 years. Concern regarding potential systemic toxic effects has limited treatment options for children younger than 2 years. We wanted to determine whether topical tacrolimus therapy is safe and effective in patients with vitiligo younger than 2 years.
Methods
The present 6-month clinical trial was conducted to evaluate the efficacy and safety of 0.03% tacrolimus in the treatment of vitiligo in children under 2 years of age. Meanwhile, serum and urine samples were collected, and liquid chromatography–mass spectrometry was performed to generate the serum and urine metabolic profile data of patients and healthy controls.
Results
The overall response rate at the sixth month, which was defined by the degree of re-pigmentation, was 100%. As revealed by blood monitoring and metabolite detection 6 months later, there was no difference between the treatment group and the control group. There is no evidence that long-term topical application of 0.03% tacrolimus ointment will cause metabolite or other physical changes in the body.
Conclusions
Tacrolimus ointment appears to be effective and safe in the treatment of vitiligo in children younger than 2 year.
Trial registration
http://www.chictr.org.cn identifier: ChiCTR 2100045920.
Impact
-
We first reported the efficacy and safety of topical application of 0.03% tacrolimus ointment in infants with vitiligo characterized by the metabolites.
-
There is no evidence that long-term topical application of 0.03% tacrolimus ointment will cause metabolite or other physical changes in the body.
-
This study provide evidence for the TCI treatment of infants with vitiligo.
Similar content being viewed by others
Introduction
Vitiligo is a common idiopathic skin disorder characterized by skin depigmentation, which is mainly caused by the destruction of cutaneous melanocytes.1,2,3 It affects 0.38–2% of all age groups in the global population.2 The pathogenesis appears to be associated with the CD8+ T cell-induced immune response, since CD8+ T and tissue-resident memory T cells (TRM) cells are recruited during both the progression and maintenance of vitiligo.4,5,6,7,8,9
Topical calcineurin inhibitors (TCIs) are recommended as the second-line therapeutics for the short-term intermittent treatment of patients with vitiligo ≥2 years.10,11,12,13 Tacrolimus, also known as FK506, is an immunomodulator that mainly functions to inhibit calcineurin activity.14 The immunosuppressive agent is commonly utilized in the patients undergoing allogeneic organ transplantation to reduce the risk of organ rejection.15,16 It is also a topical medication applied in the treatment of eczema, psoriasis, and other T cell-mediated diseases.15,16 Tacrolimus inhibits the activity of peptidylprolyl isomerase by directly binding to the immunophilin FKBP12 (FK506-binding protein).17 The FKBP12–FK506 complex interacts with and inhibits calcineurin, thus blocking T-lymphocyte signal transduction as well as the transcription and synthesis of interleukin-2 and other related cytokines.17 Significantly, tacrolimus promotes melanogenesis by regulating the growth of keratinocyte and stimulating the migration of melanocytes.18,19 Unlike the topical glucocorticoids, long-term application of TCIs shows no significant side effects in patients with atopic dermatitis (AD).20,21 In addition, it does not induce systemic immunosuppression in patients with psoriasis and vitiligo patients.22,23 As a result, TCIs application is a valuable therapeutic approach for a wide variety of other dermatologic diseases. These drugs are suitable for the intermittent long-term usage in patients, which achieve comparable effects to topical application of hormone ointment.20,24 As reported in existing studies, topical tacrolimus combined with narrow-band ultraviolet B (NB-UVB) for head and neck vitiligo attains superior therapeutic efficacy to NB-UVB treatment alone.25
The safety of tacrolimus is also evaluated in infants with dermatosis aged ≤2 years. In our recent study, we reported the efficacy of TCIs as the treatment for vitiligo in infants under 2 years of age.26 Moreover, some studies innovatively adopt urinary metabolomics to predict the potential long-term side effects due to toxicity and systemic reactions.27 However, the safety of TCIs in infants with vitiligo, and especially the systemic adverse reactions, requires further investigations.
At present, most existing studies that examine the safety of tacrolimus ointment are limited to clinical observations.15,28 In this study, we investigated the safety of topical application of 0.03% tacrolimus treatment in infants by liquid chromatography–mass spectrometry (LC/MS).
Patients and methods
The aim of the study was to explore the efficacy and tolerability of 0.03% tacrolimus cream (Protopic) in infants younger than 2 years of age. The Institutional Review Board or Ethics Committee of the participating organizations approved this study protocol. The study was performed according to the Declaration of Helsinki and Good Clinical Practices. Prior to study enrollment, the entire process was clearly explained to each patient’s parent, caregiver, or legal representative, and a concise written informed consent form was obtained.
Patients
The patient inclusion criteria were as follows: patients who were diagnosed with vitiligo by their senior physicians and did not receive systemic application of corticosteroid. Parents of the infants were informed of the potential benefits and risks related to the topical application of TCIs and signed the informed consent form. Additionally, patients with a history of spontaneous re-pigmentation of vitiligo lesions or malignant diseases or autoimmune disorders were excluded from this study.
Efficacy observation and adverse reaction observation
Vitiligo infants were managed with a standardized protocol. The skin management protocol was based on clinical practice guidelines for similar conditions. Prospective subject screening for the inclusion/exclusion criteria was completed 3 days before the baseline visit.
Patients with vitiligo were managed with a standardized protocol. To be specific, the skin management protocol was based on the clinical practice guidelines for similar conditions. Infants were treated with topical application of 0.03% tacrolimus ointment twice daily for 6 consecutive months. Afterwards, a maintenance treatment (once every 3 days) or continued treatment was implemented depending on the effect.
After the completion of treatment, all patients were subsequently followed up by the same dermatologist once a month for the following 12 months. At the same time, related data and improvement information at 6th and 12th months were recorded, respectively. In this study, the re-pigmentation rate was graded as follows: excellent (>75% re-pigmentation), good (>50–75% re-pigmentation), fair (>25–50% re-pigmentation), poor (>0–25% re-pigmentation), and no response. The effective rate was determined according to the formula: effective rate = (good case number + excellent case number)/total case number. Moreover, local adverse effects (either reported by parents or physicians) were recorded: (1) the presence or absence of scratching at the administration site by the affected child, (2) the presence or absence of erythema at the administration site, and (3) the presence or absence of scratch and blood crust at the administration site.
Sample collection and testing
From July 2021 to September 2021, blood samples and urine samples were collected from 20 infants with vitiligo who were treated with tacrolimus for 6 months. Additionally, same samples from 20 healthy infants with matched sex, height, and body weight were enrolled as controls.
Venous blood (2 mL) and urine (3 mL) were collected on an empty stomach in the early morning. Then venous blood was tested using an i2000 instrument and the supporting kit (ARCHITECT). The tacrolimus blood concentration test was performed in accordance with the i2000 standard protocols, and internal quality control (QC) was always performed.
In addition, serum and urine samples were sent to the Shanghai Meiji Company for LC/MS metabolomic analysis (Shanghai, China). A total of 40 samples were analyzed using the LC/MS platform (Thermo, Ultimate 3000LC, Q Exactive). After thawing at room temperature, 100 μL of each sample was transferred into the 1.5 mL centrifuge tubes, and then 300 μL methanol was added. After adding 10 μL internal standard, each sample was vortexed for 30 s and centrifuged at 12,000 rpm for 15 min at 4 °C. Afterwards, 200 μL supernatant was transferred into a new vial for LC/MS analysis. Chromatographic separation was conducted using a Hyper gold C18 column preheated to 40 °C. Thereafter, 10 μL prepared sample was injected and maintained at 4 °C for analysis. To obtain information on the system repeatability, QC samples were prepared by mixing and all fluid extraction aliquots were injected at regular intervals throughout the analytical process.
Metabolomic data and analysis
The data were transformed to CDF files by Thermo Scientific™ Xcalibur™ (version v3.0). Afterwards, peak picking, peak alignment, peak filtering, and peak filling were completed using the XCMS software (version v.3.4.4). Then data regarding retention time (RT), MZ, observations (samples), and peak intensity were normalized. After mean centering and unit variance scaling, principal component analysis (PCA) and (orthogonal) partial least squares discriminate analysis (O/PLS-DA) were carried out to visualize the metabolic alterations among experimental groups. Variable importance in the projection (VIP) was conducted to rank the overall contribution of each variable to the PLS-DA model, and variables with VIP > 1.0 were considered as relevant for group discrimination. Significant differences in metabolites between groups were analyzed by O/PLS-DA combined with Student’s t tests, and P values <0.05 were considered to indicate statistical significance. In the meantime, the multiple range test with corrected q values <0.05 were regarded as statistically significant.
Results
Patients and treatment
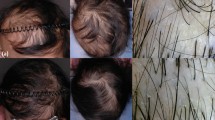
Baseline demographic data and clinical characteristics of patients and the healthy controls are summarized in Table 1. The overall response rate at the 6th month, which was defined by the degree of re-pigmentation, was 100% (Table 2 and Fig. 1). In addition, the effective rates (>50% re-pigmentation) in the tacrolimus group were 40% at the 6th month and 65% at the 12th month. No local adverse reactions were reported.
Blood concentration of tacrolimus
While measuring the blood concentration of tacrolimus, it was found that the detection values of vitiligo infants after treatment and untreated healthy controls were <1 ng/mL (data not shown), below the minimum detection value. There was no difference between the two groups.
Blood and urine LC/MS
Altogether 123,479 characteristic peaks were detected by comparing the urine mass spectrometry from 40 samples, which contained over 1020 compounds. There was no significant difference between the two groups upon PCA or further analysis by PLS-DA and OPLS-DA. Meanwhile, there was no significant metabolic difference between the two groups by differential metabolite screening test (P > 0.05) or further range test corrected q test (q < 0.05) (Fig. 2). Thus, as there were no statistically significant data for further clustering or enrichment analysis, it was impossible to conduct clustering and enrichment analyses of metabolic differences.
ML and M2 represent principal coordinate analysis (PCA) of vitiligo infants A, control group B, and QC. Orthogonal partial least squares discriminant analysis (O/PLS-DA) plot of 40 urine metabolites in comparisons of the vitiligo infants A and control group B in M3 and M4. The separation between the points relating to vitiligo and control is unclear and inseparable.
By comparing the blood mass spectrometry of the 40 samples, a total of 23,525 characteristic peaks were detected, containing about 2020 compounds. There was no statistical difference between the two groups by PCA or PLS-DA. Additionally, there was no clear separation between the points pertaining to the two groups. Thereafter, VIP metabolite screening test followed by OPLS-DA with a difference >1 was performed, and a total of 12 metabolic metabolites were found. No significant metabolic difference was detected between the two groups by range test corrected q test (q < 0.05; Fig. 3). Clustering analysis of metabolic differences was impossible. Thus, there were no statistically significant data for further counting by clustering or enrichment analysis.
ML and M2 represent principal coordinate analysis (PCA) of vitiligo infants A, control group B, and QC. Orthogonal partial least squares discriminant analysis (O/PLS-DA) plot of 40 blood metabolites in comparisons of the vitiligo infants A and control group B in M3 and M4. The separation between the points relating to vitiligo and control is unclear and inseparable.
Discussion
Topical corticosteroid preparations are one of the oldest and preferred treatments for vitiligo.12 However, in clinical practice, the vast majority of parents of vitiligo infants would have concerns about the safety and side effects of local corticosteroids. As an alternative, TCIs have been widely used to treat AD in young children currently, since they will not induce those side effects of topical steroids.11,12
However, the application of TCIs in children younger than 2 years of age was restricted in 2006 to include a boxed warning about the theoretical risk of skin cancer and lymphoma in patients treated with TCIs.29,30 As revealed by a follow-up study based on >25,000 person-years, it is unlikely that the application of topical pimecrolimus in PEER cohort for the treatment of AD is connected to an increased risk of malignancy.31 Further, there is no association between malignancy and the topical application of pimecrolimus.
The tacrolimus ointment has been shown to be efficacious and safe in treating both facial and non-facial vitiligo in the pediatric population; typically, the facial lesions responded faster than the non-facial ones.32 In this study, we found that lesions on the head and face responded better than those in other regions. As reported in a prospective pilot study on the response to treatment with tacrolimus ointment treatment for vitiligo in 30 patients in 2009, topical tacrolimus ointment is an effective and well-tolerated alternative therapy for vitiligo, especially for lesions on the head and neck.33
We found that the effective treatment time of 0.03% tacrolimus ointment for vitiligo in infants and young children was the same as that in adults, which was generally 2–2.5 months; meanwhile, good results were obtained after treatments for twice a day for 6 months. For the 16 infants treated effectively for half a year, we changed the treatment to a 3-day maintenance intermittent treatment. The complexion of infants continued to improve during the second half of the follow-up, and no recurrence occurred.
There were 22 effective treatment cases, and the effective rate was consistent with that reported in the literatures.26,34 Notably, an advantage of tacrolimus ointment over corticosteroids is that it will not cause local atrophy, dilation of capillaries, or potential adverse eye effects.35 Kanwar et al. proposed that the topical application of tacrolimus was proven to be an effective method for the treatment of vitiligo in Asian children, with a complex rate (except ineffective) of 86.4%.36 On this basis, our group was the first to include studies on infants younger than 2 years old and demonstrated TCIs as a therapeutic option for vitiligo in infants under 2 years old.
To test and verify the safety of long-term topical tacrolimus ointment, we first tested the blood concentration of the therapeutic agent. The results showed that the drug absorption and blood residue were negligible after half a year. As a classic systemic oral immunosuppressant, tacrolimus can effectively improve the prognosis of organ transplantation, and its blood concentration can reach 10 ng/mL and maintain the level for a long time.17 In our study, the final detection value of topical 0.03% tacrolimus ointment for local small areas was close to 0, and the systemic effect of long-term application was almost negligible. On the other hand, tacrolimus has been used worldwide for over 15 years to treat AD in children and adults. Available data suggested that tacrolimus is effective and well tolerated, which can improve the patient quality of life. Systemic drug absorption after tacrolimus application is negligible and unlikely to result in systemic immunosuppression. Moreover, there is currently no strong evidence of an increased rate of malignancy in treated patients with TCIs, and observational data from post-marketing surveillance studies have shown no safety concerns.37
In conclusion, we attempted to detect the changes in urine and blood metabolites in infants and young children by LC/MS. Then we analyzed and predicted the toxicity and systemic response caused by long-term drug treatment. It was found that, after 6 months of topical application, no significant change or abnormality was detected in the two groups. These results, which were obtained from the relatively stable and highly consistent infants of the recipe, proved to be credible and reliable. For blood tests performed on the same group of samples simultaneously, doubled metabolites were detected in the urine. Meanwhile, it was found through PCA and PLS-DA that there was no obvious difference between the two groups. However, 12 of these over 20,000 metabolite peaks were different. We further conducted O/PLS-DA test and multiple range R test, which confirmed that there was no difference between the two groups. Besides, there was no possible metabolic pathway or signaling pathway that might be enriched for clustering analysis. Nonetheless, more metabolomics-based long-term safety trials that use short-term clinical models to effectively predict drug toxicity and systemic response are needed.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Nahhas, A. F., Braunberger, T. L. & Hamzavi, I. H. Update on the management of vitiligo. Ski. Ther. Lett. 24, 1–6 (2019).
Taieb, A. & Picardo, M. Clinical practice. Vitiligo. N. Engl. J. Med. 360, 160–169 (2009).
Grimes, P. E. New insights and new therapies in vitiligo. JAMA 293, 730–735 (2005).
Riding, R. L. & Harris, J. E. The role of memory CD8(+) T cells in vitiligo. J. Immunol. 203, 11–19 (2019).
Fraczek, A., Owczarczyk-Saczonek, A. & Placek, W. The role of TRM cells in the pathogenesis of vitiligo-a review of the current state-of-the-art. Int. J. Mol. Sci. 21, 3552 (2020).
Wu, J., Zhou, M., Wan, Y. & Xu, A. CD8+ T cells from vitiligo perilesional margins induce autologous melanocyte apoptosis. Mol. Med. Rep. 7, 237–241 (2013).
Oyarbide-Valencia, K. et al. Therapeutic implications of autoimmune vitiligo T cells. Autoimmun. Rev. 5, 486–492 (2006).
Steitz, J., Wenzel, J., Gaffal, E. & Tuting, T. Initiation and regulation of CD8+T cells recognizing melanocytic antigens in the epidermis: implications for the pathophysiology of vitiligo. Eur. J. Cell Biol. 83, 797–803 (2004).
Wankowicz-Kalinska, A. et al. Immunopolarization of CD4+ and CD8+ T cells to Type-1-like is associated with melanocyte loss in human vitiligo. Lab. Invest. 83, 683–695 (2003).
Gawkrodger, D. J. et al. Guideline for the diagnosis and management of vitiligo. Br. J. Dermatol. 159, 1051–1076 (2008).
Lee, J. H. et al. Treatment outcomes of topical calcineurin inhibitor therapy for patients with vitiligo: a systematic review and meta-analysis. JAMA Dermatol. 155, 929–938 (2019).
Chang, H. C., Hsu, Y. P. & Huang, Y. C. The effectiveness of topical calcineurin inhibitors compared with topical corticosteroids in the treatment of vitiligo: a systematic review and meta-analysis. J. Am. Acad. Dermatol. 82, 243–245 (2020).
Nicolaidou, E., Mastraftsi, S., Tzanetakou, V. & Rigopoulos, D. Childhood vitiligo. Am. J. Clin. Dermatol. 20, 515–526 (2019).
Broen, J. C. A. & van Laar, J. M. Mycophenolate mofetil, azathioprine and tacrolimus: mechanisms in rheumatology. Nat. Rev. Rheumatol. 16, 167–178 (2020).
Baldo, A., Cafiero, M., Di, C. P. & Di, C. L. Tacrolimus ointment in the management of atopic dermatitis. Clin. Cosmet. Investig. Dermatol. 2, 1–7 (2009).
Berdoulay, A., English, R. V. & Nadelstein, B. Effect of topical 0.02% tacrolimus aqueous suspension on tear production in dogs with keratoconjunctivitis sicca. Vet. Ophthalmol. 8, 225–232 (2005).
Liu, J. et al. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66, 807–815 (1991).
Huang, H. et al. Effect and mechanism of tacrolimus on melanogenesis on A375 human melanoma cells. Chin. Med. J. 127, 2966–2971 (2014).
Fricain, J. C. et al. Mucosal pigmentation after oral lichen planus treatment with topical tacrolimus. Dermatology 210, 229–232 (2005).
Carr, W. W. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr. Drugs 15, 303–310 (2013).
Gennuso, R. et al. Lumbar intervertebral disc disease in the pediatric population. Pediatr. Neurosurg. 18, 282–286 (1992).
Guenther, L., Lynde, C. & Poulin, Y. Off-label use of topical calcineurin inhibitors in dermatologic disorders. J. Cutan. Med. Surg. 23, 27S–34S (2019).
Wong, E. & Kurian, A. Off-label uses of topical calcineurin inhibitors. Ski. Ther. Lett. 21, 8–10 (2016).
Siegfried, E. C., Jaworski, J. C., Kaiser, J. D. & Hebert, A. A. Systematic review of published trials: long-term safety of topical corticosteroids and topical calcineurin inhibitors in pediatric patients with atopic dermatitis. BMC Pediatr. 16, 75 (2016).
Li, R. et al. Effect of narrow band ultraviolet B phototherapy as monotherapy or combination therapy for vitiligo: a meta-analysis. Photodermatol. Photoimmunol. Photomed. 33, 22–31 (2017).
Hu, W. et al. Efficacy of the topical calcineurin inhibitors tacrolimus and pimecrolimus in the treatment of vitiligo in infants under 2 years of age: a randomized, open-label pilot study. Clin. Drug Investig. 39, 1233–1238 (2019).
Lee, S. J. et al. Functional interpretation of metabolomics data as a new method for predicting long-term side effects: treatment of atopic dermatitis in infants. Sci. Rep. 4, 7408 (2014).
Hanifin, J. M. et al. Efficacy and safety of tacrolimus ointment treatment for up to 4 years in patients with atopic dermatitis. J. Am. Acad. Dermatol. 53, S186–S194 (2005).
Legendre, L. et al. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta-analysis. J. Am. Acad. Dermatol. 72, 992–1002 (2015).
Tennis, P., Gelfand, J. M. & Rothman, K. J. Evaluation of cancer risk related to atopic dermatitis and use of topical calcineurin inhibitors. Br. J. Dermatol. 165, 465–473 (2011).
Margolis, D. J. et al. Association between malignancy and topical use of pimecrolimus. JAMA Dermatol. 151, 594–599 (2015).
Ho, N. et al. A double-blind, randomized, placebo-controlled trial of topical tacrolimus 0.1% vs. clobetasol propionate 0.05% in childhood vitiligo. Br. J. Dermatol. 165, 626–632 (2011).
Xu, A. E. et al. Efficacy and safety of tarcrolimus cream 0.1% in the treatment of vitiligo. Int. J. Dermatol. 48, 86–90 (2009).
Park, O. J. et al. A combination of excimer laser treatment and topical tacrolimus is more effective in treating vitiligo than either therapy alone for the initial 6 months, but not thereafter. Clin. Exp. Dermatol. 41, 236–241 (2016).
Bae, J. M. et al. Combination therapy with 308-nm excimer laser, topical tacrolimus, and short-term systemic corticosteroids for segmental vitiligo: a retrospective study of 159 patients. J. Am. Acad. Dermatol. 73, 76–82 (2015).
Kanwar, A. J., Dogra, S. & Parsad, D. Topical tacrolimus for treatment of childhood vitiligo in Asians. Clin. Exp. Dermatol. 29, 589–592 (2004).
Ohtsuki, M., Morimoto, H. & Nakagawa, H. Tacrolimus ointment for the treatment of adult and pediatric atopic dermatitis: review on safety and benefits. J. Dermatol. 45, 936–942 (2018).
Funding
This study was supported by the National Natural Science Foundation of China (81803131, 81773335 and 82003322), the Basic Public Welfare Research Project of Zhejiang Province (LGF18H110002), Natural Science Foundation of Zhejiang Province (LY18H110001), and the Health Science and Technology Projects of Hangzhou (A20220451).
Author information
Authors and Affiliations
Contributions
A.-e.X. designed the research, F.L. and J.L. performed the research, and F.L. and W.H. analyzed data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Informed consent was obtained in all cases, and protocols were approved by the scientific ethical committee of Hospital and registered in Chinese Clinical Trial Registry.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, W., Lin, F., Lei, J. et al. Impacts of exposure to topical calcineurin inhibitors on metabolism in vitiligo infants. Pediatr Res 93, 661–665 (2023). https://doi.org/10.1038/s41390-022-02133-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02133-5
This article is cited by
-
Vitiligo Treatments: Review of Current Therapeutic Modalities and JAK Inhibitors
American Journal of Clinical Dermatology (2023)