Abstract
Overall survival (OS) of pediatric patients with acute myeloid leukemia (AML) increased in recent decades. However, it remained unknown whether advances in first-line treatment, supportive care, or second-line therapy mainly contributed to this improvement. Here, we retrospectively analyzed outcome and clinical data of 1940 pediatric AML patients (younger than 18 years of age), enrolled in the population-based AML-BFM trials between 1987 and 2012. While 5-year probability of OS (pOS) increased from 49 ± 3% (1987–1992) to 76 ± 4% (2010–2012; p < 0.0001), probability of event-free survival only improved from 41 ± 3% (1987–1992) to 50 ± 2% (1993–1998; p = 0.02) after introduction of high-dose cytarabine/mitoxantrone, but remained stable since then. Non-response and relapse rates stayed constant despite intensified first-line therapy (p = 0.08 and p = 0.17). Reduced fatal bleedings and leukostasis translated into fewer early deaths (8.1%vs. 2.2%; p = 0.001). Strikingly, pOS after non-response (13 ± 5% (1987–1992) vs. 43 ± 7% (2005–2010); p < 0.0001) or relapse (19 ± 4% vs. 45 ± 4%; p < 0.0001) improved. After 1999, more relapsed or refractory patients underwent hematopoietic stem cell transplantation (HSCT) with increased pOS after HSCT (29 ± 5% (1993–1998) vs. 50 ± 4% (2005–2010); p < 0.0001). Since efficacy of salvage therapy mainly contributed to better outcome in pediatric AML, our analysis indicates that a better allocation of patients, who cannot be cured with conventional chemotherapy, to an early “salvage-like” therapy is necessary.
Similar content being viewed by others
Introduction
Prognosis of children with acute myeloid leukemia (AML) improved remarkably during the recent decades with current long-term survival rates of about 70% [1,2,3,4,5]. During this period, most international cooperative study groups have developed a treatment strategy that includes four to five courses of intensive myelosuppressive chemotherapy, mainly based on anthracyclines and cytarabine with or without hematopoietic stem cell transplantation (HSCT) [6]. This also applies to the AML-BFM trials, where several changes were introduced, concerning first-line treatment, supportive care, and second-line therapy.
The first-line treatment was intensified by introduction of courses with high-dose cytarabine or new anthracyclines or anthracycline formulations (Fig. 1a) [3, 7,8,9,10]. In the AML-BFM 87 trial, two cycles of late intensification with high-dose cytarabine (3 g/m2) and etoposide (HAE) were added after consolidation, while in the AML-BFM 93 trial, idarubicin during first induction therapy and an additional course with high-dose cytarabine (3 g/m2) and mitoxantrone (HAM) as second-induction cycle were introduced. In the AML-BFM 98 trial, a high-dose consolidation therapy (with comparable cumulative dosages of anthracyclines as before) was introduced with two short treatment cycles (cytarabine and idarubicine [AI], high-dose cytarabine (1 g/m2) and mitoxantrone (haM)). In the AML-BFM 04 trial, liposomal daunorubicin, a potentially less cardiotoxic anthracycline formulation compared to idarubicin, was introduced together with an increase of the cumulative anthracycline dose (Fig. 1a) [2, 9, 11, 12].
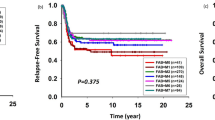
Development of overall and event-free survival from 1987–2012. a Protocol flow chart for pediatric patients with AML in AML-BFM trials from 87 until 2012. Consolidation: 6-week therapy consisting of seven different drugs: 6-thioguanine (60 mg/m2 per day, days 1–43 orally); prednisone (40 mg/m2 per day, days 1–28 orally); vincristine (1.5 mg/m2 per day, days 1, 8, 15, 22); cytarabine (75 mg/m2 per day, days 3–6, 10–13, 17–20, 24–27, 31–34, 38–41); doxorubicin (30 mg/m2 per day (AML-BFM 87 and 93)) or idarubicin (7 mg/m2 per day [AML-BFM 98], days 1, 8, 15, 22); intrathecal (age-dependent dose) cytarabine (days 1, 15, 29, 43); cyclophosphamide (500 mg/m2 per day, days 29, 43). *CNS irradiation was stopped in May 2009. Cumulative dosages of anthracycline (mg/m2) and cytarabine (g/m2) in AML-BFM trials are shown as maximal dosage given in each study. Cumulative doses were calculated as equivalent doses to daunorubicin using a ratio of 1:5 for idarubicin and mitoxantrone. ADE cytarabine (100 mg/m2), daunorubicin (60 mg/m2), etoposide (150 mg/m2), AIE cytarabine (100 mg/m2), idarubicin (12 mg/m2), etoposide (150 mg/m2), HAM high-dose cytarabine (3 g/m2), mitoxantrone (10 mg/m2), AI cytarabine (500 mg/m2), idarubicin (7 mg/m2), hAM intermediate-dose cytarabine (1 g/m2), mitoxantrone (10 mg/m2), HAE high-dose cytarabine (3 g/m2), etoposide (125 mg/m2), ADxE cytarabine (100 mg/m2), liposomal daunorubicin (80 mg/m2), etoposide (150 mg/m2), 2-CDA 2-chloro-2-deoxyadenosine (6 mg/m2), CNS central nervous system. b Development of survival per 6-year periods. Data shown as probability of EFS and OS ± SE. Kaplan–Meier curves of EFS and OS of distinct subgroups were compared using the log-rank test, shown as p value. c Development of survival per 6-year periods in patients with standard risk group [FAB M1/2 with Auer rods, FAB M4 with atypical eosinophils (M4Eo) and/or favorable cytogenetics, such as t(8;21) and/or AML1-ETO and inv(16) or t(16;16) and/or CBFB/MYH1, if there was no persistence of BM blasts (≥5%) on day 15, respectively), compared to all other patients (high-risk group). Data shown as probability of EFS and OS ± SE. Kaplan–Meier curves of EFS and OS of distinct subgroups were compared using the log-rank test, shown as p value. Standard risk: n = 88 (1987–1992); n = 174 (1993–1998); n = 151 (1999–2004); n = 148 (2005–2010); n = 39 (2011–2012). High-risk: n = 207 (1987–1992); n = 359 (1993–1998); n = 346 (1999–2004); n = 329 (2005–2010); n = 98 (2011–2012). d Overview of events (early death, death in CR, non-response, relapse, others) per 6-year periods. Data shown as probability of EFS ± SE and cumulative incidences of events. e Overview of survival and causes of deaths (caused by bleeding, infections or HSCT-related, disease-related deaths or others). Bleeding and infections have been evaluated in patients during initial disease and salvage treatment. Data shown as probability of OS ± SE and cumulative incidences of deaths. *Shorter interval for sufficient follow-up. n = 295 (1987–1992); n = 533 (1993–1998); n = 497 (1999–2004); n = 477 (2005–2010); n = 138 (2011–2012)
In parallel, agents were sequentially integrated into international guidelines to advance supportive care. Antibacterial agents, such as antipseudomonal broad-spectrum β-lactam antibiotics or carbapenems, antifungal drugs especially caspofungin or liposomal amphotericin and antiviral medications, such as aciclovir or cidofovir were approved to face high infection-related mortality [13,14,15,16,17]. With rasburicase, a drug is approved by the US Food and Drug Administration since 2002 to overcome urate nephropathy as part of tumor lysis syndrome. Already since AML-BFM 87 guidelines were made to face bleeding complications or leukostasis [18].
Lastly, trials were realized to assure a systematic and controlled approach after relapse or non-response. Although the first AML-BFM relapse study started in 1991 with low accrual rate, patients with relapse received diverse treatment regimen until 10/2001 [19]. The AML-BFM REZ 91 trial recommended a double induction of mitoxantrone and etoposid before proceeding to a 6-week consolidation and allogeneic or autologous HSCT. In study AML-BFM REZ 93, only one course of mitoxantrone and etoposid was given, followed by HAM and subsequent 6-week consolidation and either allogeneic or autologous HSCT. Both studies included a maintenance therapy [20]. Thereafter, patients were recruited to the first relapse study with appropriate patient accrual (AML-BFM REZ 97), which consisted of two blocks of reinduction with intermediate-dose cytarabine and liposomal daunorubicin, followed by allogeneic HSCT after consolidation [21]. Since 11/2001, a randomized phase III study (AML-2001/01) was opened by the International BFM (I-BFM) Study Group for pediatric patients with relapsed AML with FLAG (fludarabine, cytarabine, granulocyte-colony-stimulating factor) reinduction with or without liposomal daunorubicin, followed by a second FLAG block before proceeding to consolidation or direct to allogeneic transplantation or autologous transplantation in late relapse [22].
Although the importance of HSCT after relapse or non-response is well established, the role of HSCT in first complete remission (CR) continuously changed during the last decades [23, 24]. Several international protocols from the 1990s recommended allogeneic HSCT to all patients with matched sibling donor [25]. However, most of the recent trials use refined criteria based on risk stratification according to cytogenetic subgroups and response evaluation. Due to different definitions and indications, current transplantation rates vary from 11 to 29% [6, 25].
Thus, it remains largely unknown whether intensification of first-line treatment, better supportive care, or improved second-line therapy mainly contributed to the markedly increased overall survival (OS) in pediatric AML.
Here we present a retrospective analysis to delineate the possible causes for enhanced survival in pediatric AML, but also to reveal obstacles that may hamper further improvement. We analyzed response to first-line therapy, causes of death, survival after relapse or non-response, and the impact of HSCT in 1940 patients younger than 18 years of age with de novo AML that were enrolled in four consecutive AML-BFM trials (AML-BFM 87, AML-BFM 93, AML-BFM 98, and AML-BFM 04) between 1987 and 2012.
Patients and methods
Patients
Between 1/1987 and 12/2012, 1940 patients from 0 to 18 years of age with de novo AML in Germany, Austria (since 1993) and Switzerland were enrolled into the multicenter trials or registries of the AML-BFM studies. Any accompanying diseases that did not allow therapy according to the protocol or pre-treatment for >14 days with another intensive induction therapy resulted in exclusion of the patients from this study. We excluded patients with secondary leukemia, patients with Down syndrome myeloid leukemia or acute promyelocytic leukemia from analysis due to their unique biology and treatment. In addition, patients from Czech Republic were excluded to avoid a bias due to their late inclusion in the AML-BFM studies and the expected learning curve.
National ethics committees and institutional review boards approved this study, and patients or guardians provided written informed consent. The study was performed in accordance with the Declaration of Helsinki.
Treatment
Patients from 1987 until February 2004 were treated and risk-stratified (Supplementary Material 1) according to the respective trial protocols with increasing intensity. Detailed protocols of AML-BFM 87, AML-BFM 93, and AML-BFM 98 are described elsewhere (see Fig. 1a and introduction) [12, 26, 27]. Cumulative doses were calculated as equivalent doses to daunorubicin using a ratio of 1:5 for idarubicin and mitoxantrone as previously described [28]. Between March 2004 and April 2010, patients were treated according to the AML-BFM 04 trial (ClinicalTrials.gov Identifier: NCT00111345) [2]. Allogeneic HSCT from matched sibling donors was limited to high-risk patients in first CR, and after an amendment in 2006, allogeneic HSCT was restricted to only high-risk patients with persistent disease after second induction (bone marrow blasts ≥5%) [29, 30]. The first recommendations regarding the conditioning regimen for allogeneic SCT in first CR, consisting of busulfan and cyclophosphamide, have been included in the AML-BFM 98 trial [29]. Since April 2010, patients were treated according to best arm of the AML-BFM 04 protocol. All patients received the liposomal formulation of daunorubicin during induction therapy, and all high-risk patients received 2-chloro-2-deoxyadenosine as intensification therapy during the cytarabine/idarubicin (AI) consolidation. Randomized cranial irradiation was stopped in May 2009 [30]. Patients with relapse received diverse treatment regimen until 10/2001. For detailed information, see introduction and results.
Subgroup analysis
To further analyze the impact of time or supportive care, all high-risk patients (n = 383) of the AML-BFM 98 and AML-BFM 04 trials, who have been treated homogenously (AIE, HAM, AI, haM, HAE, maintenance, and no HSCT) have been included for a separate analysis of survival.
Definitions
The remission criteria were defined according to the Cancer and Leukemia Group B criteria at the end of intensification [31]. Probability of event-free survival (pEFS) was calculated as the time from diagnosis to the first event (relapse, death of any cause, failure to achieve remission, or secondary malignancy) or last follow-up. Failure to achieve remission was considered an event on day 0. Probability of OS (pOS) was defined as the time from the date of diagnosis to the date of death from any cause or last follow-up. Early death (ED): death within 42 days was considered early death. Patients were classified according to deaths caused by leukostasis or bleeding, infections, and others. Early relapse: all patients with relapse within 1 year after diagnosis. Medical reports of deceased patients were reviewed for evaluation of causes of deaths. More details on definitions including risk stratification are available in supplemental material.
Statistical analyses
Statistical analyses were performed with SAS version 9.03 (SAS Institute). The median follow-up was 10 years. The Kaplan–Meier method was applied to estimate probabilities of survival. EFS and OS curves of distinct subgroups were compared using the log-rank test. Cumulative incidence functions of relapse, relapse or non-response, and death in CR were constructed according to Kalbfleisch and Prentice. Competing events were all other events for pEFS: death before CR (early death) and secondary malignancy. Gray’s method was used to compare cumulative incidences. All cumulative incidence functions and survival estimates are calculated as 5-year time point. All p values are descriptive and explorative. The analysis was performed per 6-year periods (1987–1992 (n = 295); 1993–1998 (n = 533); 1999–2004 (n = 497); 2005–2010 (n = 477)). These periods reflect the recruitment times of the succeeding studies AML-BFM 87, 93, 98 and 2004. For a subset of analysis, we included patients diagnosed from 2011–2012 (n = 138), but only for investigations of initial disease as appropriate follow-up is required. For a subset of analysis, patients were grouped depending on age, always defined as age of diagnosis. Only patients with allogeneic HSCT have been included in the analyses of transplantation. For all analyses of risk group stratification, the following definition was used retrospectively: standard risk indicates FAB M1/2 with Auer rods, FAB M4 with atypical eosinophils (M4Eo) and/or favorable cytogenetics, such as t(8;21) and/or AML1-ETO and inv(16) or t(16;16) and/or CBFB/MYH1, if there was no persistence of bone marrow (BM) blasts (≥5%) on day 15. FLT3-ITD positivity was not considered. All others were classified as high-risk patients. The database lock for this analysis was set at 1 July 2016. Of the 1940 patients, 962 (576, 335) were alive at the time of data freeze and followed for at least 5 (10, 15) years.
Results
Development of overall and event-free survival from 1987 to 2012
The first-line treatment was intensified stepwise from 1987 to 2012 with increased cumulative doses of cytarabine (maximum 47 g/m2 in AML-BFM 98) and anthracycline (maximum 510 mg/m2 in AML-BFM 04; Fig. 1a). The 5-year pOS of 1940 patients registered in the AML-BFM studies showed a continuous, and significant improvement from 1987–1992 (49 ± 3%) to 2011–2012 (76 ± 4%; p < 0.0001; Fig. 1b). In contrast, the pEFS only increased significantly from 41 ± 3% (1987–1992) to 50 ± 2% (1993–1998; p = 0.02) after introduction of high-dose cytarabine/mitoxantrone, but remained stable since then (p = 0.21) (Fig. 1b). Thus, there is an increasing gap between pEFS and pOS (8%, (1987–1992) vs. 21%, (2011–2012)) (Fig. 1b). Subgroup analysis revealed that both, pEFS (p = 0.0029) and pOS (p < 0.0001), improved in patients with high-risk AML, while in patients with standard risk AML, only pOS increased (p = 0.0044) (Fig. 1c). Young patients (younger than 2 years of age) showed a benefit in pOS and patients older than 13 years of age showed a constant improvement of pEFS and pOS (Supplementary Figure 1A).
The impact of first-line treatment
Intensification of first-line therapy resulted in an insignificant reduction of the cumulative incidences of relapse (32 ± 3% vs. 25 ± 4%, p = 0.17) and non-response (16 ± 2% vs. 12 ± 3%, p = 0.08) from 1987 till 2012 (Fig. 1d and Supplementary Table 1). However, the rate of non-response significantly dropped between 1987–1992 and 1993–1998 (p = 0.025) (Supplementary Table 1). This drop was associated with an improved pEFS and coincided with the introduction of HAM (Fig. 1a). Moreover, there was a relevant change in causes of death during the first 6 weeks after initial diagnosis (early death). Whereas in earlier trials, deaths due to bleeding or leukostasis were most predominant, they were rarely found in later trials (1987–1992 vs. 2011–2012; p = 0.0041; Fig. 2a). The total number of early deaths decreased from 8.1% (1987–1992) to 2.2% in the most recent cohort (2011–2012; p = 0.001; Fig. 2a). The early death rate due to infections varied (1987–1992 vs. 2011–2012; p = 0.53; Fig. 2a). We also observed a trend toward fewer deaths in CR (p = 0.17) (Fig. 2b), mostly due to fewer infection-related deaths.
First-line therapy. a Causes of early death from 1987–2012. Data shown as cumulative incidences of deaths ± SE. Gray’s method was used to compare cumulative incidences, shown as p value. Death due to other reasons or inconclusive causes of deaths includes three patients who have been delayed in diagnosis and did not receive treatment in time. n = 295 (1987–1992); n = 533 (1993–1998); n = 497 (1999–2004); n = 477 (2005–2010). b Causes of death during CR from 1987–2012. Data shown as cumulative incidences of deaths ± SE. Gray’s method was used to compare cumulative incidences. n = 295 (1987–1992); n = 533 (1993–1998); n = 497 (1999–2004); n = 477 (2005–2010). c Development of survival after allogeneic HSCT in first CR from 1987–2010. Analysis of survival (pOS and pEFS ± SE) after HSCT in 6-year periods (1987–1992: n = 18; 1993–1998: n = 52; 1999–2004: n = 69; 2005–2010: n = 43). Kaplan–Meier curves of EFS and OS of distinct subgroups were compared using the log-rank test, shown as p value. d Development of survival of all patients without allogeneic HSCT in first CR from 1987–2010. Analysis of survival (pOS and pEFS ± SE) in 6-year periods (1987–1992: n = 277; 1993–1998: n = 481; 1999–2004: n = 428; 2005–2010: n = 434). Kaplan–Meier curves of EFS and OS of distinct subgroups were compared using the log-rank test, shown as p value
The allogeneic transplantation rate in first CR varied over time (6.1% (1987–1992) vs. 9.0% (2005–2010); Supplementary Table 2) and the pOS after allogeneic HSCT increased after 1993 from 60 ± 10% (1993–1998) to 81 ± 6% (2005–2010; p = 0.08) and pEFS from 52 ± 7% (1993–1998) to 74 ± 7% (2005–2010; p = 0.06; Fig. 2c). Of note, in patients without allogeneic HSCT in first CR, the pEFS remained stable from 1993 until 2010 (49 ± 2% vs. 48 ± 2%, p = 0.89) (Fig. 2d).
The impact of salvage treatment on improved overall survival
The increasing gap between pEFS and pOS after first-line therapy indicates that the survival after an event was improved. Therefore, we analyzed the impact of salvage therapy on the improved pOS (detailed information on protocols in Fig. 3a and introduction). Notably, the 5-year pOS of patients after relapse improved from 19 ± 4% (1987–1992) to 45 ± 4% (2005–2010; p < 0.0001), with the highest increase from 1999–2004 to 2005–2010 (28 ± 3% vs. 45 ± 4%; p = 0.0003) (Fig. 3d). The 5-year pEFS after relapse showed a comparable development from 19 ± 4% (1987–1992) to 40 ± 4% (2005–2010) (p < 0.0001) (Fig. 3b). The same significant increase in pEFS and pOS was observed in patients with non-response (Fig. 3c, e). The improvement coincided with the start of the relapsed AML 2001/01 I-BFM study. The changes in salvage therapy resulted in a considerable reduction of disease-related deaths in patients with relapse (64.1 ± 5.0 vs. 30.7 ± 3.8%; p < 0.0001) or non-response (52.1 ± 7.4 vs. 25.5 ± 6.5%; p = 0.0038) (Fig. 3d, e). Young patients had the highest benefit from improved salvage therapy (younger than 2 years of age: 14 ± 5% (1987–1992) vs. 56 ± 7% (2005–2010); p < 0.0001 and 2–9 years of age: 13 ± 5% (1987–1992) vs. 45 ± 6% (2005–2010); p < 0.0001) (Supplementary Figure 1B).
Non-response and relapse. a Protocol flow chart of trials AML-BFM REZ 91, AML-BFM REZ 93, AML-BFM REZ 97, and Relapsed AML 2001/01. HAM high-dose cytarabine, mitoxantrone, SCT stem cell transplantation, MITOX mitoxantrone, E etoposid, A cytarabine (intermediate dose), Dx liposomal daunorubicine, FLAG fludarabine, cytarabine, G-CSF, Consol. consolidation consisting of thioguanine and low-dose cytarabine for 6 weeks. +Low patient recruitment due to alternative regimens, no treatment or palliative care. *Autologous SCT if no suitable matched allogeneic donor available in late relapses. Analysis of pEFS ± SE after relapse (b) and their cumulative incidences of deaths ± SE and pOS ± SE from 1987–2010 (d). n = 97 (1987–1992); n = 159 (1993–1998); n = 172 (1999–2004); n = 156 (2005–2010). Analysis of pEFS ± SE after non-response (c) and their cumulative incidences of deaths ± SE and pOS ± SE (e). n = 48 (1987–1992); n = 56 (1993–1998); n = 46 (1999–2004); n = 47 (2005–2010). Causes of deaths are evaluated for bleeding, infections, SCT-related, disease-related, or others. b–e Kaplan–Meier curves of EFS and OS of distinct subgroups were compared using the log-rank test, shown as p value. BFM Berlin Frankfurt Münster, CNS central nervous system
Infectious-related deaths or bleeding-related deaths were significantly reduced in non-responders (20.8 ± 5.9% vs. 6.4 ± 3.6%; p = 0.042 or 10.4 ± 4.5% vs. 0.0 ± 0.0%; p = 0.024), while it was insignificantly reduced after relapse (infections 9.3 ± 3.0% vs. 6.5 ± 2.0%; p = 0.39 or bleeding 2.1 ± 1.5% vs. 0.0 ± 0.0%; p = 0.07) (Fig. 3d, e).
The lower disease-related mortality after relapse or non-response was accompanied by a higher HSCT-related mortality (5.2 ± %2.3 vs. 16.9 ± 0.3%; p = 0.0075; Fig. 3d and 2.1 ± 2.1% vs. 21.3 ± 6.1%; p = 0.004; Fig. 3e), which can be explained by the higher number of patients that underwent HSCT (relapse: 22% (1987–1992) vs. 75% (2005–2010), p < 0.0001; non-response: 19% vs. 85%, p < 0.0001, Supplementary Table 3). Still, patients undergoing allogeneic HSCT after first relapse or non-response showed an improved survival, most notably after 1999 (29 ± 5% (1993–1998) vs. 50 ± 4% (2005–2010); p < 0.0001; Fig. 4a). The improvement over time was most prominent in patients with early relapse (0 ± 0% (1987–1992) vs. 47 ± 6% (2005–2010); p = 0.015), while the survival increased insignificantly in patients with late relapse (42 ± 13% (1987–1992) vs. 55 ± 7% (2005–2010); p = 0.41; Fig. 4b). In contrast, patients of both groups showed a dismal survival without allogeneic HSCT that did not change over time. Patients with an initial standard cytogenetic risk profile showed a particular increase in pOS after HSCT after first relapse (34 ± 9% (1987–1998) vs. 60 ± 7% (1999–2010); p = 0.016; Fig. 4c), while for high-risk patients the pOS remained poor (35 ± 6% (1987–1998) vs. 42 ± 4% (1999–2010); p = 0.15; Fig. 4c).
Allogeneic HSCT after relapse or non-response. Development of survival after HSCT from 1987–2010. a Analysis of pOS and pEFS ± SE after allogeneic HSCT in 6-year periods in patients with relapse or non-response. (1987–1992: n = 30; 1993–1998: n = 92; 1999–2004: n = 129; 2005–2010: n = 156). b Analysis of 5-year pOS ± SE in patients with early (<1 year of date of diagnosis) or late relapse (>1 year of date of diagnosis) with (+) and without (−) allogeneic HSCT. Early relapse with HSCT n = 5 (1987–1992); n = 31 (1993–1998); n = 46 (1999–2004); n = 63 (2005–2010), early relapse without HSCT n = 35 (1987–1992); n = 52 (1993–1998); n = 43 (1999–2004); n = 18 (2005–2010). Late relapse with HSCT n = 16 (1987–1992); n = 41 (1993–1998); n = 55 (1999–2004); n = 53 (2005–2010), late relapse without HSCT n = 40 (1987–1992); n = 28 (1993–1998); n = 20 (1999–2004); n = 20 (2005–2010). c Analysis of 5-year pOS ± SE after allogeneic HSCT in relapsed AML of standard risk or high-risk AML. Data shown in two time periods ((1987–1998) and (1999–2010)). Standard risk group indicates FAB M1/2 with Auer rods, FAB M4 with atypical eosinophils (M4Eo) and/or favorable cytogenetics, such as t(8;21) and/or AML1-ETO and inv(16) or t(16;16) and/or CBFB/MYH1, if there was no persistence of BM blasts (≥5%) on day. All others were classified as high-risk group. a–c EFS and OS curves of distinct subgroups were compared using the log-rank test, shown as p value
Supportive care—a subgroup analysis
To analyze the role of supportive care for the improved survival independent of changes made to the treatment protocol, we performed a subgroup analysis of homogenously treated patients enrolled over a period of 12 years (n = 383). This was possible as one arm of all high-risk patients of the AML-BFM 98 and AML-BFM 04 trials was equal (Supplementary Figure 2A). We excluded patients with HSCT to avoid a bias through probably variable criteria for selection of HSCT and different procedures.
Patient characteristics were comparable between both groups (Supplementary Table 4). The survival analysis showed that pEFS, but also pOS did not change significantly (Supplementary Figure 2B). Similarly, despite of expanded supportive care recommendations (Supplementary Material 2) treatment-related mortality, including infectious deaths, did not change between both study populations (Supplementary Figure 2C).
Discussion
Long-term survival of children with AML has significantly improved within the recent decades [1,2,3,4,5]. In this retrospective analysis of 1940 pediatric patients treated within the AML-BFM protocols between 1987 and 2012, we deciphered the contribution of intensified first-line treatment, supportive care and salvage therapy to this success (summarized in Fig. 5). We showed that intensification of first-line therapy translated into an unsteady and insignificant improvement of pEFS over the past 25 years, which is mainly explained by a failure to significantly reduce the relapse or non-response rate. Only the major drug intensification in the AML-BFM 93 study (i.e., introduction of HAM) resulted in a significant increase of pEFS. Still, one must consider that improving the outcome was not only the aim of the AML-BFM trials. In the AML-BFM 98 and 04 trials, it could be shown in a randomized non-inferiority study that reduced cranial irradiation does not result in a survival disadvantage [30].
Pediatric AML from 1987–2010. Fractions of pediatric patients are classified regarding their status quo (alive in relapse, alive in first CR, alive after non-response, death after relapse or non-response and other deaths such as early death or death in CR) from 1987–2010. n = 295 (1987–1992); n = 533 (1993–1998); n = 497 (1999–2004); n = 477 (2005–2010)
The better pOS despite a nearly constant rate of non-response and relapse since 1993 can be regarded as major advance of salvage therapy. The remarkably reduced disease-related mortality in patients with non-response and relapse assures nowadays a survival rate of 45% in these patients. This development, especially in young children, patients with early relapse or with an initial standard risk cytogenetic profile, is most probably due to a systematic treatment of patients after relapse or non-response and higher survival rates after transplantation. In particular, the better outcome after 1999 coincided with the introduction of the international relapse protocol and rising transplantation rates. Of note, survival after relapse already started to increase in patients enrolled in AML-Rez 97, but the patient accrual was still low. Interestingly, autologous SCT was only performed in a relevant percentage of patients in 1987–1992 (n = 21; 21.6% of all patients with first relapse). In the following years, this procedure was only rarely used in a relapse setting (n = 10; 6.3% (1993–1998), n = 2; 1.2% (1999–2004), n = 1; 0.6% (2004–2010)).
The impact of supportive care is more difficult to assess. An analysis with a subset of patients of this study, who have been treated with identical chemotherapy over 12 years, failed to show a significant change of pEFS and treatment-related mortality despite of more advanced recommendations. However, the small number of patients within this analysis and the retrospective design may limit this subgroup analysis. Different aspects of this study may illustrate the importance of advanced supportive care in pediatric AML. The reduced early death rate, especially due to bleeding or leukostasis, reflects improved supporting procedures and guidelines for cytoreductive therapy in case of hyperleukocytosis. Moreover, advances in supportive care may be the basis for the increase of patients with relapse proceeding to HSCT and for the improved survival after HSCT. This can be exemplified by a decreased infectious-related mortality especially in patients with non-response. A recent study reported a significantly reduced infectious-related mortality in AML-BFM 04 compared to historic data of the AML-BFM 93 study [14, 32], especially in the first 42 days (early deaths group) or after achieving CR, which is in accordance with our study [14, 33]. Of note, in contrast to the previous study, we included treatment-related mortality during initial and relapse treatment. Yet, the significantly reduced infectious-related mortality in the aforementioned subgroups is counterbalanced by an increasing HSCT-related mortality. HSCT-related mortality is becoming increasingly important due to higher percentage of patients, being transplanted after extensive treatment in non-response or relapse, constituting a major challenge for the upcoming years. This means that critically sick patients are nowadays eligible for HSCT, which were previously excluded from this procedure. Nevertheless, the remarkably increasing survival rate of patients with HSCT underlines the progress that was achieved. However, prospective randomized studies in pediatric AML are difficult to perform and thereby the exact value for patients with HSCT in first CR is still unclear [23,24,25]. Since 2010 a prospective non-randomized, multinational, and on-site monitored BFM-HSCT trial for pediatric AML has been initiated aiming for therapy optimization. It includes patients with second CR or primary refractory disease, analyzing the role of matched sibling donors vs. matched unrelated donors, the effectivity of “FLAMSA” and in a subgroup of patients the relevance of haploidentical transplantation (EudraCT: 2007–004517–34, NCT00606723).
The trial could harmonize transplantation-related center effects and will better define the role of HSCT in the treatment of pediatric AML. Results of this trial will be separately reported.
In conclusion, we identified three main factors, which were responsible for improved outcome. First, treatment intensification from AML-BFM 87 to the AML-BFM 93 study resulted in a significant reduction of non-responders and relapses, which was not seen by treatment intensification in later studies. Second, the efficacy of second-line therapy including HSCT, which was consequently applied since 2001 according to an international study protocol, was most successful to improve outcome in pediatric AML during the last 25 years. Third, better supportive care contributed to reduce the early death rate and partly infectious-related deaths.
These findings of our retrospective analysis have important implications for the future. Further intensification of anthracycline- and cytarabine-based chemotherapy will most likely not result in a major reduction of relapse or non-response. The development of new classes of innovative drugs or immunotherapy in first-line therapy is required to enhance response and reduce the risk of relapse. Better stratification of patients based on their individual genetic risk and measurements of minimal residual disease would help to quickly allocate patients to a “salvage-like” therapy that cannot be cured with conventional first-line chemotherapy only. This should be ideally proven in a prospective study with long-term follow-up and monitoring of quality of life. And finally, further improvements in supportive care are urgently needed to keep pace with intensified toxicity, especially after HSCT.
References
Abrahamsson J, Forestier E, Heldrup J, Jahnukainen K, Jonsson OG, Lausen B, et al. Response-guided induction therapy in pediatric acute myeloid leukemia with excellent remission rate. J Clin Oncol. 2011;29:310–5.
Creutzig U, Zimmermann M, Bourquin JP, Dworzak MN, Fleischhack G, Graf N, et al. Randomized trial comparing liposomal daunorubicin with idarubicin as induction for pediatric acute myeloid leukemia: results from Study AML-BFM 2004. Blood. 2013;122:37–43.
Gibson BE, Wheatley K, Hann IM, Stevens RF, Webb D, Hills RK, et al. Treatment strategy and long-term results in paediatric patients treated in consecutive UK AML trials. Leukemia. 2005;19:2130–8.
Pession A, Masetti R, Rizzari C, Putti MC, Casale F, Fagioli F, et al. Results of the AIEOP AML 2002/01 multicenter prospective trial for the treatment of children with acute myeloid leukemia. Blood. 2013;122:170–8.
Rubnitz JE, Inaba H, Dahl G, Ribeiro RC, Bowman WP, Taub J, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11:543–52.
Zwaan CM, Kolb EA, Reinhardt D, Abrahamsson J, Adachi S, Aplenc R, et al. Collaborative efforts driving progress in pediatric acute myeloid leukemia. J Clin Oncol. 2015;33:2949–62.
Ravindranath Y, Chang M, Steuber CP, Becton D, Dahl G, Civin C, et al. Pediatric Oncology Group (POG) studies of acute myeloid leukemia (AML): a review of four consecutive childhood AML trials conducted between 1981 and 2000. Leukemia. 2005;19:2101–16.
Woods WG, Neudorf S, Gold S, Sanders J, Buckley JD, Barnard DR, et al. A comparison of allogeneic bone marrow transplantation, autologous bone marrow transplantation, and aggressive chemotherapy in children with acute myeloid leukemia in remission. Blood. 2001;97:56–62.
Creutzig U, Ritter J, Zimmermann M, Reinhardt D, Hermann J, Berthold F, et al. Improved treatment results in high-risk pediatric acute myeloid leukemia patients after intensification with high-dose cytarabine and mitoxantrone: results of Study Acute Myeloid Leukemia-Berlin-Frankfurt-Munster 93. J Clin Oncol. 2001;19:2705–13.
Creutzig U, Zimmermann M, Dworzak MN, Ritter J, Schellong G, Reinhardt D. Development of a curative treatment within the AML-BFM studies. Klin Padiatr. 2013;225(Suppl 1):S79–86.
Creutzig U, Ritter J, Zimmermann M, Schellong G. Does cranial irradiation reduce the risk for bone marrow relapse in acute myelogenous leukemia? Unexpected results of the Childhood Acute Myelogenous Leukemia Study BFM-87. J Clin Oncol. 1993;11:279–86.
Creutzig U, Zimmermann M, Lehrnbecher T, Graf N, Hermann J, Niemeyer CM, et al. Less toxicity by optimizing chemotherapy, but not by addition of granulocyte colony-stimulating factor in children and adolescents with acute myeloid leukemia: results of AML-BFM 98. J Clin Oncol. 2006;24:4499–506.
Sung L, Aplenc R, Alonzo TA, Gerbing RB, Lehrnbecher T, Gamis AS. Effectiveness of supportive care measures to reduce infections in pediatric AML: a report from the Children’s Oncology Group. Blood. 2013;121:3573–7.
Bochennek K, Hassler A, Perner C, Gilfert J, Schoning S, Klingebiel T, et al. Infectious complications in children with acute myeloid leukemia: decreased mortality in multicenter trial AML-BFM 2004. Blood Cancer J. 2016;6:e382.
Robinson PD, Lehrnbecher T, Phillips R, Dupuis LL, Sung L. Strategies for empiric management of pediatric fever and neutropenia in patients with cancer and hematopoietic stem-cell transplantation recipients: a systematic review of randomized trials. J Clin Oncol. 2016;34:2054–60.
Lehrnbecher T, Phillips R, Alexander S, Alvaro F, Carlesse F, Fisher B, et al. Guideline for the management of fever and neutropenia in children with cancer and/or undergoing hematopoietic stem-cell transplantation. J Clin Oncol. 2012;30:4427–38.
Sung L, Robinson P, Treister N, Baggott T, Gibson P, Tissing W, et al. Guideline for the prevention of oral and oropharyngeal mucositis in children receiving treatment for cancer or undergoing haematopoietic stem cell transplantation. BMJ Support Palliat Care. 2015;7:7–16.
Creutzig U, Ritter J, Budde M, Sutor A, Schellong G. Early deaths due to hemorrhage and leukostasis in childhood acute myelogenous leukemia. Associations with hyperleukocytosis and acute monocytic leukemia. Cancer. 1987;60:3071–9.
Sander A, Zimmermann M, Dworzak M, Fleischhack G, von Neuhoff C, Reinhardt D, et al. Consequent and intensified relapse therapy improved survival in pediatric AML: results of relapse treatment in 379 patients of three consecutive AML-BFM trials. Leukemia. 2010;24:1422–8.
Stahnke K, Boos J, Bender-Gotze C, Ritter J, Zimmermann M, Creutzig U. Duration of first remission predicts remission rates and long-term survival in children with relapsed acute myelogenous leukemia. Leukemia. 1998;12:1534–8.
Reinhardt D, Hempel G, Fleischhack G, Schulz A, Boos J, Creutzig U. Liposomal daunorubicine combined with cytarabine in the treatment of relapsed/refractory acute myeloid leukemia in children. Klin Padiatr. 2002;214:188–94.
Kaspers GJ, Zimmermann M, Reinhardt D, Gibson BE, Tamminga RY, Aleinikova O, et al. Improved outcome in pediatric relapsed acute myeloid leukemia: results of a randomized trial on liposomal daunorubicin by the International BFM Study Group. J Clin Oncol. 2013;31:599–607.
Niewerth D, Creutzig U, Bierings MB, Kaspers GJ. A review on allogeneic stem cell transplantation for newly diagnosed pediatric acute myeloid leukemia. Blood. 2010;116:2205–14.
Bleakley M, Lau L, Shaw PJ, Kaufman A. Bone marrow transplantation for paediatric AML in first remission: a systematic review and meta-analysis. Bone Marrow Transplant. 2002;29:843–52.
Hasle H. A critical review of which children with acute myeloid leukaemia need stem cell procedures. Br J Haematol. 2014;166:23–33.
Creutzig U, Zimmermann M, Ritter J, Reinhardt D, Hermann J, Henze G, et al. Treatment strategies and long-term results in paediatric patients treated in four consecutive AML-BFM trials. Leukemia. 2005;19:2030–42.
Creutzig U, Zimmermann M, Bourquin JP, Dworzak MN, von Neuhoff C, Sander A, et al. Second induction with high-dose cytarabine and mitoxantrone: different impact on pediatric AML patients with t(8;21) and with inv(16). Blood. 2011;118:5409–15.
A systematic collaborative overview of randomized trials comparing idarubicin with daunorubicin (or other anthracyclines) as induction therapy for acute myeloid leukaemia. AML Collaborative Group. Br J Haematol. 1998;103:100–9.
Klusmann JH, Reinhardt D, Zimmermann M, Kremens B, Vormoor J, Dworzak M, et al. The role of matched sibling donor allogeneic stem cell transplantation in pediatric high-risk acute myeloid leukemia: results from the AML-BFM 98 study. Haematologica. 2012;97:21–9.
Creutzig U, Zimmermann M, Bourquin JP, Dworzak MN, Fleischhack G, von Neuhoff C, et al. CNS irradiation in pediatric acute myleoid leukemia: equal results by 12 or 18 Gy in studies AML-BFM98 and 2004. Pediatr Blood Cancer. 2011;57:986–92.
Cheson BD, Cassileth PA, Head DR, Schiffer CA, Bennett JM, Bloomfield CD, et al. Report of the National Cancer Institute-sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol. 1990;8:813–9.
Lehrnbecher T, Varwig D, Kaiser J, Reinhardt D, Klingebiel T, Creutzig U. Infectious complications in pediatric acute myeloid leukemia: analysis of the prospective multi-institutional clinical trial AML-BFM 93. Leukemia. 2004;18:72–7.
Creutzig U, Zimmermann M, Reinhardt D, Dworzak M, Stary J, Lehrnbecher T. Early deaths and treatment-related mortality in children undergoing therapy for acute myeloid leukemia: analysis of the multicenter clinical trials AML-BFM 93 and AML-BFM 98. J Clin Oncol. 2004;22:4384–93.
Acknowledgements
We are grateful to patients for participation and data managers in all hospitals and offices for their efforts in this study. We thank J.E. Müller for data management. This work was supported by the Deutsche Krebshilfe e.V. J.-H.K. receives funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 714226).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J.-P.B. has consulting or advisory roles for Amgen and Roche. T.K. has consulting or advisory roles for Loxo and Novartis. T.L. receives honoraria from Gilead, Merk Sharp & Dome, Astella, Basilea, and has consulting or advisory roles and participates in speakers’ bureaus, including travel accommodation and other expenses from Gilead, Merk Sharp & Dome, Astella. T.L. receives research funding from Gilead. D.R. has consulting or advisory roles for Roche, Celgene, Hexal, Pfizer, Novartis, Boehringer and receives research funding from Celgene. D.R. received travel, accommodation or other expenses from Jazz Pharmaceuticals and Griffols. The remaining authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. If you remix, transform, or build upon this article or a part thereof, you must distribute your contributions under the same license as the original. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/.
About this article
Cite this article
Rasche, M., Zimmermann, M., Borschel, L. et al. Successes and challenges in the treatment of pediatric acute myeloid leukemia: a retrospective analysis of the AML-BFM trials from 1987 to 2012. Leukemia 32, 2167–2177 (2018). https://doi.org/10.1038/s41375-018-0071-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-018-0071-7
This article is cited by
-
Development and validation of a promising 5-gene prognostic model for pediatric acute myeloid leukemia
Molecular Biomedicine (2024)
-
Genomic DNA-based measurable residual disease monitoring in pediatric acute myeloid leukemia: unselected consecutive cohort study
Leukemia (2024)
-
Panel-based RNA fusion sequencing improves diagnostics of pediatric acute myeloid leukemia
Leukemia (2024)
-
Effective methods for bulk RNA-seq deconvolution using scnRNA-seq transcriptomes
Genome Biology (2023)
-
RUNX1 mutation has no prognostic significance in paediatric AML: a retrospective study of the AML-BFM study group
Leukemia (2023)