Abstract
Background
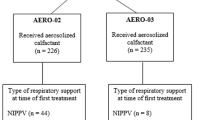
The aerosolized calfactant decreased the need for intubation in neonates with respiratory distress syndrome (AERO-02 trial).
Objective
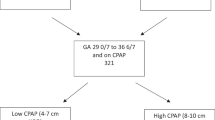
To determine the oxygenation response to aerosolized calfactant among infants born 28 0/7–36 6/7 weeks with RDS in the AERO-02 trial.
Methods
Trends in hourly fraction of oxygen (FiO2), mean airway pressure (MAP) and respiratory severity score (RSS) were compared between the aerosolized calfactant (AC) and usual care (UC) groups from time of randomization for 72 h.
Results
A total of 353 subjects were included in the study. FiO2, MAP, and RSS were lower in the UC group. FiO2 decrease was seen after the first aerosolized calfactant dose.
Conclusion
FiO2, MAP, and RSS were lower in the UC group. This is likely due to early and higher rate of liquid surfactant administration in the UC group. Decrease in FiO2 was noted in the AC group after the first aerosolization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, [DK], upon request.
References
Polin RA, Carlo WA. Committee on Fetus and Newborn; American Academy of Pediatrics. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics. 2014;133:156–63.
Sweet DG, Carnielli V, Greisen G, Hallman M, Klebermass-Schrehof K, Ozek E, et al. European Consensus guidelines on the management of respiratory distress syndrome - 2022 update. Neonatology. 2023;120:3–23.
Ng EH, Shah V, Fetus and Newborn Committee. Canadian Paediatric Society. Guidelines for surfactant replacement therapy in neonates. Paediatr Child Health. 2021;26:35–49.
Isayama T, Chai-Adisaksopha C, McDonald SD. Noninvasive ventilation with vs without early surfactant to prevent chronic lung disease in preterm infants: a systematic review and meta-analysis. JAMA Pediatr. 2015;169:731–9.
Herting E, Härtel C, Göpel W. Less invasive surfactant administration: best practices and unanswered questions. Curr Opin Pediatr. 2020;32:228–34.
Kakkilaya V, Gautham SK. Should less invasive surfactant administration (LISA) become routine practice in US neonatal units? Pediatr Res. 2022;19:1–11.
Zapata HA, Fort P, Roberts KD, Kaluarachchi DC, Guthrie SO. Surfactant administration through laryngeal or supraglottic airways (SALSA): a viable method for low-income and middle-income countries. Front Pediatr. 2022;10:853831.
Brasher M, Raffay TM, Cunningham MD, Abu Jawdeh EG. Aerosolized surfactant for preterm infants with respiratory distress syndrome. Children. 2021;8:493.
Pillow JJ, Minocchieri S. Innovation in surfactant therapy II: surfactant administration by aerosolization. Neonatology. 2012;101:337–44.
Lewis JF, Ikegami M, Jobe AH, Tabor B. Aerosolized surfactant treatment of pre- term lambs. J Appl Physiol. 1991;70:869–76.
Schermuly RT, Gunther A, Weissmann N, Ghofrani HA, Seeger W, Grimminger F, et al. Differential impact of ultra- sonically nebulized versus tracheal-instilled surfactant on ventilation-perfusion (VA/Q) mismatch in a model of acute lung injury. Am J Respir Crit Care Med. 2000;161:152–9.
Rey-Santano C, Mielgo VE, Andres L, Ruiz-del-Yerro E, Valls-i-Soler A, Murgia X. Acute and sustained effects of aerosolized vs. bolus surfactant therapy in premature lambs with respiratory distress syndrome. Pediatr Res. 2013;73:639–46.
Jorch G, Weller E, Murlat A, Hentschel R. Feasibility study on nebulization of bovine surfactant (SF-RI 1) by pharyngeal continuous positive airway pressure (CPAP). Biol. Neonate. 1994;66.
Berggren E, Liljedahl M, Winbladh B, Andreasson B, Curstedt T, Robertson B, et al. Pilot study of nebulized surfactant therapy for neonatal respiratory distress syndrome. Acta Pediatr. 2000;89:460–4.
Dani C, Talosi G, Piccinno A, Maria Ginocchio V, Balla G, Lavizzari A, et al. A randomized, controlled trial to investigate the efficacy of nebulized poractant alfa in premature babies with respiratory distress syndrome. J Pediatr. 2022;246:40–7.e5.
Cummings JJ, Gerday E, Minton S, Katheria A, Albert G, Flores-Torres J, et al. Aerosolized calfactant for newborns with respiratory distress: a randomized trial. Pediatrics. 2020;146:e20193967.
Kielt MJ, Logan JW, Backes CH, Conroy S, Reber KM, Shepherd EG, et al. Noninvasive respiratory severity indices predict adverse outcomes in bronchopulmonary dysplasia. J Pediatr. 2022;242:129–36.e2.
Klein JP, Logan B, Harhoff M, Anderson PK. Analyzing survival curves at a fixed point in time. Stat Med. 2007;26:4505–19.
Anderson PK, Perme MP. Pseudo-observations in survival analysis. Stat Methods Med Res. 2010;19:71–99.
R Core Team. (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Robillard F, Alarie Y, Dagenais-Perusse P, Baril E, Guilbeault A. Micro- aerosol administration of synthetic beta-gamma-dipalmitoyl-L-alpha- lecithin in the respiratory distress syndrome. A preliminary report. Can Med Assoc J. 1964;90:55–7.
Chu J, Clements JA, Cotton EK, Klaus MH, Sweet AY, Tooley WH. Neo- natal pulmonary ischemia: clinical and physiologic studies. Pediatrics. 1967;40:709–82.
Finer NN, Allen Merritt T, Bernstein G, Job L, Mazela J, Segal R. An open label, pilot study of aerosurf combined with nCPAP to prevent RDS in preterm neonates. J Aerosol Med Pulm Drug Deliv. 2010;23:303–9.
Sood BG, Cortez J, Kolli M, Sharma A, Delaney-Black V, Chen X. Aerosolized surfactant in neonatal respiratory distress syndrome: phase I study. Early Hum Dev. 2019;134:19–25.
Sood B, Thomas R, Delaney-Black V, Xin Y, Sharma A, Chen X. Aerosolized beractant in neonatal respiratory distress syndrome: a randomized fixed-dose parallel-arm phase II trial. Pulm Pharm Ther. 2021;66:101986.
Minocchieri S, Berry CA, Pillow JJ, CureNeb Study Team. Nebulised surfactant to reduce severity of respiratory distress: a blinded, parallel, randomized controlled trial. Arch Dis Child Fetal Neonatal Ed. 2019;104:F313–9.
Gaertner VD, Thomann J, Bassler D, Ruegger CM. Surfactant nebulization to prevent intubation in preterm infants: a systematic review and meta-analysis. Pediatrics. 2021;148:e2021052504.
Kim K, Choi SQ, Zell ZA, Squires TM, Zasadzinski JA. Effect of cholesterol nanodomains on monolayer morphology and dynamics. Proc Natl Acad Sci USA. 2013;110:E3054–60.
Hatch LD, Grubb PH, Lea AS, Walsh WF, Markham MH, Maynordet PO, et al. Endotracheal intubation in neonates: a prospective study of adverse safety events in 162 infants. J Pediatr. 2016;168:62–6.
Trembath A, Hornik CP, Clark R, Smith PB, Daniels J, Laughon M, et al. Comparative effectiveness of surfactant preparations in premature infants. J Pediatr. 2013;163:955–60.
Acknowledgements
All AERO-02 study investigators, Melissa Brown, Corey Commaroto, ONY Biotech Inc.
Author information
Authors and Affiliations
Contributions
DK: designed the study, drafted the project proposal, obtained data, drafted and approved final manuscript as submitted. HAZ: involved in designing of the study, drafted the project proposal, reviewed and approved final manuscript as submitted. HLB: involved in designing of the study, drafted the project proposal, reviewed and approved final manuscript as submitted. MRL: involved in designing of the study, did the data analysis, reviewed and approved final manuscript as submitted. PF: involved in designing of the study, reviewed and approved final manuscript as submitted. SOG: involved in designing of the study, reviewed and approved final manuscript as submitted. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
PF and SG have worked for and received payments from ONY Biotech in the past. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaluarachchi, D.C., Zapata, H.A., Becker, H.L. et al. Response to aerosolized calfactant in infants with respiratory distress syndrome; a post-hoc analysis of AERO-02 trial. J Perinatol 43, 998–1003 (2023). https://doi.org/10.1038/s41372-023-01717-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01717-1