Abstract
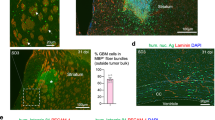
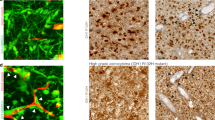
Glioblastoma multiforme (GBM) are highly invasive and angiogenic malignancies with a median survival time from diagnosis of <15 months. Previous work has revealed robust overexpression of fibronectin (FN) mRNA in GBM, although immunohistochemical staining of FN in these tumors is typically associated with the angiogenic vasculature. Here we sought to examine the expression of tumor cell FN and address its possible involvement in the invasive phenotype of GBM. We found that FN was expressed and assembled into fibrillar arrays in human tumors and in established GBM lines. Cultured cells spontaneously formed dense cellular networks and spheroid-like domes. Depletion of FN by targeted-short hairpin RNA expression disrupted matrix assembly and multicellular network organization by exerting profound effects on cell adhesion and motility. Although FN depletion enhanced persistent directional migration of single cells, it compromised collective invasion of spheroids through a laminin-rich matrix and sensitized cells to ionizing radiation. In orthotopic grafts, FN depletion significantly reduced tumor growth and angiogenesis. Together our results show that FN produced by the tumor cells has a role in GBM pathophysiology and they provide insights into the implications that targeting FN interactions may have for combating this dreaded disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Wen PY, Kesari S . Malignant gliomas in adults. N Engl J Med 2008; 359: 492–507.
Claes A, Idema AJ, Wesseling P . Diffuse glioma growth: a guerilla war. Acta Neuropathol. 2007; 114: 443–458.
Venstrom KA, Reichardt LF . Extracellular matrix. 2: role of extracellular matrix molecules and their receptors in the nervous system. FASEB J 1993; 7: 996–1003.
Rauch U . Extracellular matrix components associated with remodeling processes in brain. Cell Mol Life Sci 2004; 61: 2031–2045.
Zamecnik J . The extracellular space and matrix of gliomas. Acta Neuropathol 2005; 110: 435–442.
Lal A, Lash AE, Altschul SF, Velculescu V, Zhang L, McLendon RE et al. A public database for gene expression in human cancers. Cancer Res 1999; 59: 5403–5407.
Colin C, Baeza N, Bartoli C, Fina F, Eudes N, Nanni I et al. Identification of genes differentially expressed in glioblastoma versus pilocytic astrocytoma using suppression subtractive hybridization. Oncogene 2006; 25: 2818–2826.
Castellani P, Borsi L, Carnemolla B, Biro A, Dorcaratto A, Viale GL et al. Differentiation between high- and low-grade astrocytoma using a human recombinant antibody to the extra domain-B of fibronectin. Am J Pathol 2002; 161: 1695–1700.
Mahesparan R, Read TA, Lund-Johansen M, Skaftnesmo KO, Bjerkvig R, Engebraaten O . Expression of extracellular matrix components in a highly infiltrative in vivo glioma model. Acta Neuropathol 2003; 105: 49–57.
Ohnishi T, Hiraga S, Izumoto S, Matsumura H, Kanemura Y, Arita N et al. Role of fibronectin-stimulated tumor cell migration in glioma invasion in vivo: clinical significance of fibronectin and fibronectin receptor expressed in human glioma tissues. Clin Exp Metastasis 1998; 16: 729–741.
Yuan L, Siegel M, Choi K, Khosla C, Miller CR, Jackson EN et al. Transglutaminase 2 inhibitor, KCC009, disrupts fibronectin assembly in the extracellular matrix and sensitizes orthotopic glioblastomas to chemotherapy. Oncogene 2007; 26: 2563–2573.
Friedlander DR, Zagzag D, Shiff B, Cohen H, Allen JC, Kelly PJ et al. Migration of brain tumor cells on extracellular matrix proteins in vitro correlates with tumor type and grade and involves alphaV and beta1 integrins. Cancer Res 1996; 56: 1939–1947.
Ohnishi T, Arita N, Hiraga S, Taki T, Izumoto S, Fukushima Y et al. Fibronectin-mediated cell migration promotes glioma cell invasion through chemokinetic activity. Clin Exp Metastasis 1997; 15: 538–546.
Hynes RO . The extracellular matrix: not just pretty fibrils. Science 2009; 326: 1216–1219.
Cseh B, Fernandez-Sauze S, Grall D, Schaub S, Doma E, Van Obberghen-Schilling E . Autocrine fibronectin directs matrix assembly and crosstalk between cell-matrix and cell-cell adhesion in vascular endothelial cells. J Cell Sci 2010; 123 (Pt 22): 3989–3999.
Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008; 455: 1061–1068.
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010; 17: 98–110.
Singh P, Carraher C, Schwarzbauer JE . Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol 2010; 26: 397–419.
Casey RC, Burleson KM, Skubitz KM, Pambuccian SE, Oegema TR Jr., Ruff LE et al. Beta 1-integrins regulate the formation and adhesion of ovarian carcinoma multicellular spheroids. Am J Pathol 2001; 159: 2071–2080.
Salmenpera P, Kankuri E, Bizik J, Siren V, Virtanen I, Takahashi S et al. Formation and activation of fibroblast spheroids depend on fibronectin-integrin interaction. Exp Cell Res 2008; 314: 3444–3452.
Burden-Gulley SM, Qutaish MQ, Sullivant KE, Lu H, Wang J, Craig SE et al. Novel cryo-imaging of the glioma tumor microenvironment reveals migration and dispersal pathways in vivid three-dimensional detail. Cancer Res 71: 5932–5940.
Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A et al. Tensional homeostasis and the malignant phenotype. Cancer cell 2005; 8: 241–254.
Ulrich TA, de Juan Pardo EM, Kumar S . The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res 2009; 69: 4167–4174.
Bissell MJ, Hall HG, Parry G . How does the extracellular matrix direct gene expression? J Theor Biol 1982; 99: 31–68.
Spencer VA, Xu R, Bissell MJ . Gene expression in the third dimension: the ECM-nucleus connection. J Mammary Gland Biol Neoplasia 2010; 15: 65–71.
Nam JM, Onodera Y, Bissell MJ, Park CC . Breast cancer cells in three-dimensional culture display an enhanced radioresponse after coordinate targeting of integrin alpha5beta1 and fibronectin. Cancer Res 2010; 70: 5238–5248.
Desgrosellier JS, Cheresh DA . Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 2010; 10: 9–22.
Tabatabai G, Weller M, Nabors B, Picard M, Reardon D, Mikkelsen T et al. Targeting integrins in malignant glioma. Target Oncol 2010; 5: 175–181.
Farber K, Synowitz M, Zahn G, Vossmeyer D, Stragies R, van Rooijen N et al. An alpha5beta1 integrin inhibitor attenuates glioma growth. Mol Cell Neurosci 2008; 39: 579–585.
Maglott A, Bartik P, Cosgun S, Klotz P, Ronde P, Fuhrmann G et al. The small alpha5beta1 integrin antagonist, SJ749, reduces proliferation and clonogenicity of human astrocytoma cells. Cancer Res 2006; 66: 6002–6007.
Martinkova E, Maglott A, Leger DY, Bonnet D, Stiborova M, Takeda K et al. alpha5beta1 integrin antagonists reduce chemotherapy-induced premature senescence and facilitate apoptosis in human glioblastoma cells. Int J Cancer 2010; 127: 1240–1248.
Hendrix MJ, Seftor EA, Hess AR, Seftor RE . Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer 2003; 3: 411–421.
Yue WY, Chen ZP . Does vasculogenic mimicry exist in astrocytoma? J Histochem Cytochem 2005; 53: 997–1002.
Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe'er J et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol 1999; 155: 739–752.
Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 2010; 468: 824–828.
Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature 2010; 468: 829–833.
Bellail AC, Hunter SB, Brat DJ, Tan C, Van Meir EG . Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol 2004; 36: 1046–1069.
Bernstein JJ, Woodard CA . Glioblastoma cells do not intravasate into blood vessels. Neurosurgery 1995; 36: 124–132.
Maubant S, Saint-Dizier D, Boutillon M, Perron-Sierra F, Casara PJ, Hickman JA et al. Blockade of alpha v beta3 and alpha v beta5 integrins by RGD mimetics induces anoikis and not integrin-mediated death in human endothelial cells. Blood 2006; 108: 3035–3044.
Kelm JM, Timmins NE, Brown CJ, Fussenegger M, Nielsen LK . Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol Bioeng 2003; 83: 173–180.
Vouret-Craviari V, Boulter E, Grall D, Matthews C, Van Obberghen-Schilling E . ILK is required for the assembly of matrix-forming adhesions and capillary morphogenesis in endothelial cells. J Cell Sci 2004; 117 (Pt 19): 4559–4569.
Stern P, Astrof S, Erkeland SJ, Schustak J, Sharp PA, Hynes RO . A system for Cre-regulated RNA interference in vivo. Proc Natl Acad Sci USA 2008; 105: 13895–13900.
Argyriou AA, Antonacopoulou A, Iconomou G, Kalofonos HP . Treatment options for malignant gliomas, emphasizing towards new molecularly targeted therapies. Crit Rev Oncol Hematol 2009; 69: 199–210.
Ricard C, Stanchi F, Rodriguez T, Amoureux MC, Rougon G, Debarbieux F . Dynamic quantitative intravital imaging of glioblastoma progression reveals a lack of correlation between tumor growth and blood vessel density. Plos One 2013, (in press).
Acknowledgements
We thank Jean-François Michiels and Philippe Paquis (Pathology and Neurosurgery Departments of the Nice CHU) for their collaboration. We also thank Botond Cseh, Nathalie Baeza-Kallee, Clément Ricard, Sandeep Gopal and Juliette Thariat for valuable discussions, cell lines, help with intracranial xenograft experiments and statistical analyses. Patrick Stern and Richard Hynes are kindly acknowledged for providing the lentiviral vector, Aurélie Rossin for assistance with cytometry, Sebastien Schaub (PRISM Platform, CNRS-UMR6543) and the PICSIL Platform (CNRS-6216) for expert help with image analyses. Serge Marcie and Jean-Louis Fischel (Centre A. Lacassagne) are gratefully acknowledged for helping with irradiation experiments, and Arnaud Borderie, Sandrine Destree, Coralie Hagnere et Bérengère Szczépaniak for immunohistochemistry. Financial support for this work was provided by the French National Institute of Cancer (INCa, RS018), the French Association of Cancer Research (ARC 4965 to EVO-S and 5095 to GR), the National Agency for Research (JCC PathoVisu3dyn to FD), la Fondation de Recherche sur le Cerveau to FD, Canceropole PACA and l’Agence Nationale pour la Recherche (ANR Jeunes Chercheurs, ‘GLIOMIRSTEM’ to TV).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Serres, E., Debarbieux, F., Stanchi, F. et al. Fibronectin expression in glioblastomas promotes cell cohesion, collective invasion of basement membrane in vitro and orthotopic tumor growth in mice. Oncogene 33, 3451–3462 (2014). https://doi.org/10.1038/onc.2013.305
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.305
Keywords
This article is cited by
-
miR-138-5p-mediated HOXD11 promotes cell invasion and metastasis by activating the FN1/MMP2/MMP9 pathway and predicts poor prognosis in penile squamous cell carcinoma
Cell Death & Disease (2022)
-
L1CAM is required for early dissemination of fallopian tube carcinoma precursors to the ovary
Communications Biology (2022)
-
High Expression of Fibronectin 1 Predicts a Poor Prognosis in Glioblastoma
Current Medical Science (2022)
-
Proteomics analysis identifies PEA-15 as an endosomal phosphoprotein that regulates α5β1 integrin endocytosis
Scientific Reports (2021)
-
A vascularized tumoroid model for human glioblastoma angiogenesis
Scientific Reports (2021)