Abstract
Metastatic breast adenocarcinomas display activation signatures for signaling pathways that trigger cell motility and tissue invasion. Here, we report that the adaptor protein transducer of Cdc42-dependent actin assembly-1 (Toca-1) is expressed in highly invasive breast cancers and regulates their metastatic phenotypes. We show that Toca-1 localizes to the filamentous actin-rich core of invadopodial protrusions actively degrading the extracellular matrix (ECM). Toca-1 colocalizes with Cortactin, and we show that this interaction is mediated by the SH3 domain of Toca-1. Stable knockdown (KD) of Toca-1 expression in MDA-MB-231 cells led to a significant defect in epidermal growth factor (EGF)-induced cell migration and invasion. Toca-1 KD cells also showed significant defects in EGF- and Src-induced ECM digestion and formation of invadopodial membrane protrusions. To test the role of Toca-1 in metastasis, we achieved stable Toca-1 KD in both human and rat metastatic breast adenocarcinoma cell lines. Orthotopic tumor xenografting of control and Toca-1 KD cells in natural-killer /B-/T-cell-deficient mice revealed a significant defect in spontaneous lung metastases with Toca-1 silencing in vivo. In contrast, no defects in primary tumor growth or lung seeding following tail vein injection of Toca-1 KD cells was observed, suggesting that Toca-1 functions at an early step in the dissemination of metastatic breast tumor cells. Taken together, our results identify Toca-1 as a proinvasive protein in breast adenocarcinoma and a potential therapeutic target to limit tumor metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
de Boer M, van Dijck JA, Bult P, Borm GF, Tjan-Heijnen VC . Breast cancer prognosis and occult lymph node metastases, isolated tumor cells, and micrometastases. JNCI 2010; 102: 410–425.
Van Trappen PO, Pepper MS . Lymphatic dissemination of tumour cells and the formation of micrometastases. Lancet Oncol 2002; 3: 44–52.
Rakha EA, El-Sayed ME, Reis-Filho J, Ellis IO . Patho-biological aspects of basal-like breast cancer. Breast Cancer Res Treat 2009; 113: 411–422.
Rakha EA, Reis-Filho JS, Ellis IO . Basal-like breast cancer: a critical review. J Clin Oncol 2008; 26: 2568–2581.
Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol 2006; 24: 5652–5657.
Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 2004; 10: 5367–5374.
Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res 2005; 65: 5278–5283.
Hochgrafe F, Zhang L, O’Toole SA, Browne BC, Pinese M, Porta Cubas A et al. Tyrosine phosphorylation profiling reveals the signaling network characteristics of Basal breast cancer cells. Cancer Res 2010; 70: 9391–9401.
Corkery B, Crown J, Clynes M, O’Donovan N . Epidermal growth factor receptor as a potential therapeutic target in triple-negative breast cancer. Ann Oncol 2009; 20: 862–867.
Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res 2004; 64: 7022–7029.
Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim HD, Giampieri S et al. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell 2008; 15: 813–828.
Xue C, Wyckoff J, Liang F, Sidani M, Violini S, Tsai KL et al. Epidermal growth factor receptor overexpression results in increased tumor cell motility in vivo coordinately with enhanced intravasation and metastasis. Cancer Res 2006; 66: 192–197.
Gimona M, Buccione R, Courtneidge SA, Linder S . Assembly and biological role of podosomes and invadopodia. Curr Op Cell Biol 2008; 20: 235–241.
Stylli SS, Kaye AH, Lock P . Invadopodia: at the cutting edge of tumour invasion. J Clin Neurosci 2008; 15: 725–737.
Frittoli E, Palamidessi A, Disanza A, Scita G . Secretory and endo/exocytic trafficking in invadopodia formation: the MT1-MMP paradigm. Eur J Cell Biol 2010; 90: 108–114.
Luo M, Guan JL . Focal adhesion kinase: a prominent determinant in breast cancer initiation, progression and metastasis. Cancer Lett 2010; 289: 127–139.
Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M et al. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol 2005; 168: 441–452.
Ho HY, Rohatgi R, Lebensohn AM, Le M, Li J, Gygi SP et al. Toca-1 mediates Cdc42-dependent actin nucleation by activating the N-WASP-WIP complex. Cell 2004; 118: 203–216.
Aspenstrom P, Fransson A, Richnau N . Pombe Cdc15 homology proteins: regulators of membrane dynamics and the actin cytoskeleton. Trends Biochem Sci 2006; 31: 670–679.
Chitu V, Stanley ER . Pombe Cdc15 homology (PCH) proteins: coordinators of membrane-cytoskeletal interactions. Trends Cell Biol 2007; 17: 145–156.
Fricke R, Gohl C, Bogdan S . The F-BAR protein family Actin’ on the membrane. Comm Integrat Biol 2010; 3: 89–94.
Suetsugu S . The direction of actin polymerization for vesicle fission suggested from membranes tubulated by the EFC/F-BAR domain protein FBP17. FEBS Lett 2009; 583: 3401–3404.
Takano K, Toyooka K, Suetsugu S . EFC/F-BAR proteins and the N-WASP-WIP complex induce membrane curvature-dependent actin polymerization. EMBO J 2008; 27: 2817–2828.
Bu W, Lim KB, Yu YH, Chou AM, Sudhaharan T, Ahmed S . Cdc42 interaction with N-WASP and Toca-1 regulates membrane tubulation, vesicle formation and vesicle motility: implications for endocytosis. PloS One 2010; 5: e12153.
Bu W, Chou AM, Lim KB, Sudhaharan T, Ahmed S . The Toca-1-N-WASP complex links filopodial formation to endocytosis. J Biol Chem 2009; 284: 11622–11636.
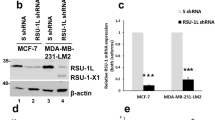
Hu J, Mukhopadhyay A, Craig AW . Transducer of Cdc42-dependent actin assembly promotes epidermal growth factor-induced cell motility and invasiveness. J Biol Chem 2011; 286: 2261–2272.
Fricke R, Gohl C, Dharmalingam E, Grevelhorster A, Zahedi B, Harden N et al. Drosophila Cip4/Toca-1 integrates membrane trafficking and actin dynamics through WASP and SCAR/WAVE. Curr Biol 2009; 19: 1429–1437.
Giuliani C, Troglio F, Bai Z, Patel FB, Zucconi A, Malabarba MG et al. Requirements for F-BAR proteins TOCA-1 and TOCA-2 in actin dynamics and membrane trafficking during Caenorhabditis elegans oocyte growth and embryonic epidermal morphogenesis. PLoS Genet 2009; 5: e1000675.
Aspenstrom P . Formin-binding proteins: modulators of formin-dependent actin polymerization. Biochim Biophys Acta 2010; 1803: 174–182.
Aspenstrom P, Richnau N, Johansson AS . The diaphanous-related formin DAAM1 collaborates with the Rho GTPases RhoA and Cdc42, CIP4 and Src in regulating cell morphogenesis and actin dynamics. Exp Cell Res 2006; 312: 2180–2194.
Lizarraga F, Poincloux R, Romao M, Montagnac G, Le Dez G, Bonne I et al. Diaphanous-related formins are required for invadopodia formation and invasion of breast tumor cells. Cancer Res 2009; 69: 2792–2800.
Sun X, Li C, Zhuang C, Gilmore WC, Cobos E, Tao Y et al. Abl interactor 1 regulates Src-Id1-matrix metalloproteinase 9 axis and is required for invadopodia formation, extracellular matrix degradation and tumor growth of human breast cancer cells. Carcinogenesis 2009; 30: 2109–2116.
Hu J, Mukhopadhyay A, Truesdell P, Chander H, Mukhopadhyay UK, Mak AS et al. Cdc42-interacting protein 4 is a Src substrate that regulates invadopodia and invasiveness of breast tumors by promoting MT1-MMP endocytosis. J Cell Sci 2011; 124 (Pt 10): 1739–1751.
Pichot CS, Arvanitis C, Hartig SM, Jensen SA, Bechill J, Marzouk S et al. Cdc42-interacting protein 4 promotes breast cancer cell invasion and formation of invadopodia through activation of N-WASp. Cancer Res 2010; 70: 8347–8356.
Yamamoto H, Sutoh M, Hatakeyama S, Hashimoto Y, Yoneyama T, Koie T et al. Requirement for FBP17 in invadopodia formation by invasive bladder tumor cells. J Urol 2011; 185: 1930–1938.
Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC . Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res 2006; 66: 3034–3043.
Webb BA, Eves R, Mak AS . Cortactin regulates podosome formation: roles of the protein interaction domains. Exp Cell Res 2006; 312: 760–769.
Desmarais V, Yamaguchi H, Oser M, Soon L, Mouneimne G, Sarmiento C et al. N-WASP and cortactin are involved in invadopodium-dependent chemotaxis to EGF in breast tumor cells. Cell Motil Cytoskeleton 2009; 66: 303–316.
Bravo-Cordero JJ, Oser M, Chen X, Eddy R, Hodgson L, Condeelis J . A novel spatiotemporal RhoC activation pathway locally regulates cofilin activity at invadopodia. Curr Biol 2011; 21: 635–644.
Le Devedec SE, van Roosmalen W, Maria N, Grimbergen M, Pont C, Lalai R et al. An improved model to study tumor cell autonomous metastasis programs using MTLn3 cells and the Rag2(−/−) gammac (−/−) mouse. Clin Exp Met 2009; 26: 673–684.
Rucci N, Recchia I, Angelucci A, Alamanou M, Del Fattore A, Fortunati D et al. Inhibition of protein kinase c-Src reduces the incidence of breast cancer metastases and increases survival in mice: implications for therapy. J Pharmacol Exp Ther 2006; 318: 161–172.
Itoh T, Erdmann KS, Roux A, Habermann B, Werner H, De Camilli P . Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev Cell 2005; 9: 791–804.
Tsujita K, Suetsugu S, Sasaki N, Furutani M, Oikawa T, Takenawa T . Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis. J Cell Biol 2006; 172: 269–279.
Shimada A, Takano K, Shirouzu M, Hanawa-Suetsugu K, Terada T, Toyooka K et al. Mapping of the basic amino-acid residues responsible for tubulation and cellular protrusion by the EFC/F-BAR domain of pacsin2/Syndapin II. FEBS Lett 2010; 584: 1111–1118.
Campellone KG, Siripala AD, Leong JM, Welch MD . Membrane-deforming proteins play distinct roles in actin pedestal biogenesis by enterohemorrhagic E.coli. J Biol Chem 2012; 287: 20613–20624.
Wang W, Goswami S, Lapidus K, Wells AL, Wyckoff JB, Sahai E et al. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer Res 2004; 64: 8585–8594.
Wang W, Wyckoff JB, Goswami S, Wang Y, Sidani M, Segall JE et al. Coordinated regulation of pathways for enhanced cell motility and chemotaxis is conserved in rat and mouse mammary tumors. Cancer Res 2007; 67: 3505–3511.
Rao Y, Ma Q, Vahedi-Faridi A, Sundborger A, Pechstein A, Puchkov D et al. Molecular basis for SH3 domain regulation of F-BAR-mediated membrane deformation. Proc Natl Acad Sci USA 2010; 107: 8213–8218.
Wang Q, Navarro MV, Peng G, Molinelli E, Goh SL, Judson BL et al. Molecular mechanism of membrane constriction and tubulation mediated by the F-BAR protein Pacsin/Syndapin. Proc Natl Acad Sci USA 2009; 106: 12700–12705.
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007; 13 (15 Pt 1): 4429–4434.
Nurnberg A, Kitzing T, Grosse R . Nucleating actin for invasion. Nature Rev 2011; 11: 177–187.
Devy L, Huang L, Naa L, Yanamandra N, Pieters H, Frans N et al. Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis. Cancer Res 2009; 69: 1517–1526.
Eckert MA, Yang J . Targeting invadopodia to block breast cancer metastasis. Oncotarget 2011; 2: 562–568.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408.
Steffen A, Le Dez G, Poincloux R, Recchi C, Nassoy P, Rottner K et al. MT1-MMP-dependent invasion is regulated by TI-VAMP/VAMP7. Curr Biol 2008; 18: 926–931.
Valsecchi ME, McDonald M, Brody JB, Hyslop T, Freydin B, Yeo CJ et al. Epidermal growth factor receptor and insulinlike growth factor 1 receptor expression predict poor survival in pancreatic ductal adenocarcinoma. Cancer (e-pub ahead of print 15 November 2011; doi:10.1002/cncr.26661).
Acknowledgements
We greatfully acknowledge Stephanie Everingham, Jinghui Hu and Alka Mukhopadhyay for help in preparing key reagents, and Chandra Tayade for providing mice. Thanks to Sohail Ahmed, Phillipe Chavrier and Alan Mak for providing plasmids, and Leda Raptis and Doris Germain for providing cell lines. This research is supported by a grant from Canadian Breast Cancer Foundation (Ontario, Canada) to AWBC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Chander, H., Truesdell, P., Meens, J. et al. Transducer of Cdc42-dependent actin assembly promotes breast cancer invasion and metastasis. Oncogene 32, 3080–3090 (2013). https://doi.org/10.1038/onc.2012.317
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.317
Keywords
This article is cited by
-
Himalayan flora: targeting various molecular pathways in lung cancer
Medical Oncology (2023)
-
High levels of unfolded protein response component CHAC1 associates with cancer progression signatures in malignant breast cancer tissues
Clinical and Translational Oncology (2022)
-
Prognostic significance of CHAC1 expression in breast cancer
Molecular Biology Reports (2022)
-
Homeostatic membrane tension constrains cancer cell dissemination by counteracting BAR protein assembly
Nature Communications (2021)
-
High formin binding protein 17 (FBP17) expression indicates poor differentiation and invasiveness of ductal carcinomas
Scientific Reports (2020)