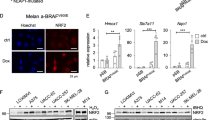
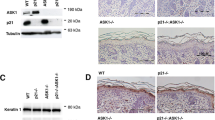
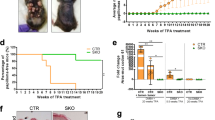
Abstract
Mutations or deletions in the cyclin-dependent kinase inhibitor p16INK4A are associated with multiple cancer types, but are more commonly found in melanoma tumors and associated with familial melanoma predisposition. Although p16 is thought to function as a tumor suppressor by negatively regulating the cell cycle, it remains unclear why the genetic compromise of p16 predisposes to melanoma over other cancers. Here we describe a novel role for p16 in regulating oxidative stress in several cell types, including melanocytes. Expression of p16 was rapidly upregulated following ultraviolet-irradiation and in response to H2O2-induced oxidative stress in a p38 stress-activated protein kinase-dependent manner. Knockdown of p16 using small interfering RNA increased intracellular reactive oxygen species (ROS) and oxidative (8-oxoguanine) DNA damage, which was further enhanced by H2O2 treatment. Elevated ROS levels were also observed in p16-depleted human keratinocytes and in whole skin and dermal fibroblasts from Cdkn2a-deficient mice. Aberrant ROS and p38 signaling in Cdkn2a-deficient fibroblasts was normalized by expression of exogenous p16. The effect of p16 depletion on ROS was not recapitulated by the knockdown of retinoblastoma protein (Rb) and did not require Rb. Finally, p16-mediated suppression of ROS could not be attributed to the potential effects of p16 on cell cycle phase. These findings suggest a potential alternate Rb-independent tumor-suppressor function of p16 as an endogenous regulator of carcinogenic intracellular oxidative stress. Compared with keratinocytes and fibroblasts, we also found increased susceptibility of melanocytes to oxidative stress in the context of p16 depletion, which may explain why the compromise of p16 predisposes to melanoma over other cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Alcorta DA, Xiong Y, Phelps D, Hannon G, Beach D, Barrett JC . (1996). Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci USA 93: 13742–13747.
Becker TM, Haferkamp S, Dijkstra MK, Scurr LL, Frausto M, Diefenbach E et al. (2009). The chromatin remodelling factor BRG1 is a novel binding partner of the tumor suppressor p16INK4a. Mol Cancer 8: 4.
Becker TM, Rizos H, Kefford RF, Mann GJ . (2001). Functional impairment of melanoma-associated p16(INK4a) mutants in melanoma cells despite retention of cyclin-dependent kinase 4 binding. Clin Cancer Res 7: 3282–3288.
Bochar DA, Wang L, Beniya H, Kinev A, Xue Y, Lane WS et al. (2000). BRCA1 is associated with a human SWI/SNF-related complex: linking chromatin remodeling to breast cancer. Cell 102: 257–265.
Bockholt SM, Burridge K . (1995). An examination of focal adhesion formation and tyrosine phosphorylation in fibroblasts isolated from src-, fyn-, and yes- mice. Cell Adhes Commun 3: 91–100.
Bowen AR, Hanks AN, Allen SM, Alexander A, Diedrich MJ, Grossman D . (2003). Apoptosis regulators and responses in human melanocytic and keratinocytic cells. J Invest Dermatol 120: 48–55.
Cathcart R, Schwiers E, Ames BN . (1983). Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem 134: 111–116.
Choi BY, Choi HS, Ko K, Cho YY, Zhu F, Kang BS et al. (2005). The tumor suppressor p16(INK4a) prevents cell transformation through inhibition of c-Jun phosphorylation and AP-1 activity. Nat Struct Mol Biol 12: 699–707.
Chung JS, Lee SB, Park SH, Kang ST, Na AR, Chang TS et al. (2009). Mitochondrial reactive oxygen species originating from Romo1 exert an important role in normal cell cycle progression by regulating p27(Kip1) expression. Free Radic Res 43: 729–737.
Cicchillitti L, Fasanaro P, Biglioli P, Capogrossi MC, Martelli F . (2003). Oxidative stress induces protein phosphatase 2A-dependent dephosphorylation of the pocket proteins pRb, p107, and p130. J Biol Chem 278: 19509–19517.
Cotter MA, Thomas J, Cassidy P, Robinette K, Jenkins N, Florell SR et al. (2007). N-acetylcysteine protects melanocytes against oxidative stress/damage and delays onset of ultraviolet-induced melanoma in mice. Clin Cancer Res 13: 5952–5958.
Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H et al. (2005). Distinct sets of genetic alterations in melanoma. N Engl J Med 353: 2135–2147.
Dhomen N, Reis-Filho JS, da Rocha Dias S, Hayward R, Savage K, Delmas V et al. (2009). Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell 15: 294–303.
Eiberger W, Volkmer B, Amouroux R, Dherin C, Radicella JP, Epe B . (2008). Oxidative stress impairs the repair of oxidative DNA base modifications in human skin fibroblasts and melanoma cells. DNA Repair (Amst) 7: 912–921.
Flores JF, Walker GJ, Glendening JM, Haluska FG, Castresana JS, Rubio MP et al. (1996). Loss of the p16INK4a and p15INK4b genes, as well as neighboring 9p21 markers, in sporadic melanoma. Cancer Res 56: 5023–5032.
Gilchrest BA, Park HY, Eller MS, Yaar M . (1996). Mechanisms of ultraviolet light-induced pigmentation. Photochem Photobiol 63: 1–10.
Goldstein AM, Chan M, Harland M, Gillanders EM, Hayward NK, Avril MF et al. (2006). High-risk melanoma susceptibility genes and pancreatic cancer, neural system tumors, and uveal melanoma across GenoMEL. Cancer Res 66: 9818–9828.
Grammatico P, Maresca V, Roccella F, Roccella M, Biondo L, Catricala C et al. (1998). Increased sensitivity to peroxidizing agents is correlated with an imbalance of antioxidants in normal melanocytes from melanoma patients. Exp Dermatol 7: 205–212.
Gray-Schopfer VC, Cheong SC, Chong H, Chow J, Moss T, Abdel-Malek ZA et al. (2006). Cellular senescence in naevi and immortalisation in melanoma: a role for p16? Br J Cancer 95: 496–505.
Haferkamp S, Becker TM, Scurr LL, Kefford RF, Rizos H . (2008). p16INK4a-induced senescence is disabled by melanoma-associated mutations. Aging Cell 7: 733–745.
Haferkamp S, Scurr LL, Becker TM, Frausto M, Kefford RF, Rizos H . (2009). Oncogene-induced senescence does not require the p16(INK4a) or p14ARF melanoma tumor suppressors. J Invest Dermatol 129: 1983–1991.
Herrling T, Jung K, Fuchs J . (2006). Measurements of UV-generated free radicals/reactive oxygen species (ROS) in skin. Spectrochim Acta A Mol Biomol Spectrosc 63: 840–845.
Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K et al. (2006). Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med 12: 446–451.
Leikam C, Hufnagel A, Schartl M, Meierjohann S . (2008). Oncogene activation in melanocytes links reactive oxygen to multinucleated phenotype and senescence. Oncogene 27: 7070–7082.
Lukas J, Parry D, Aagaard L, Mann DJ, Bartkova J, Strauss M et al. (1995). Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature 375: 503–506.
Macip S, Igarashi M, Fang L, Chen A, Pan ZQ, Lee SW et al. (2002). Inhibition of p21-mediated ROS accumulation can rescue p21-induced senescence. EMBO J 21: 2180–2188.
Meyskens Jr FL, Farmer P, Fruehauf JP . (2001). Redox regulation in human melanocytes and melanoma. Pigment Cell Res 14: 148–154.
Mooi WJ, Peeper DS . (2006). Oncogene-induced cell senescence—halting on the road to cancer. N Engl J Med 355: 1037–1046.
Naidu S, Vijayan V, Santoso S, Kietzmann T, Immenschuh S . (2009). Inhibition and genetic deficiency of p38 MAPK up-regulates heme oxygenase-1 gene expression via Nrf2. J Immunol 182: 7048–7057.
Pashaei S, Li L, Zhang H, Spencer HJ, Schichman SA, Fan CY et al. (2008). Concordant loss of heterozygosity of DNA repair gene, hOGG1, in melanoma in situ and atypical melanocytic hyperplasia. J Cutan Pathol 35: 525–531.
Pavel S, van Nieuwpoort F, van der Meulen H, Out C, Pizinger K, Cetkovska P et al. (2004). Disturbed melanin synthesis and chronic oxidative stress in dysplastic naevi. Eur J Cancer 40: 1423–1430.
Piepkorn M . (2000). The expression of p16(INK4a), the product of a tumor suppressor gene for melanoma, is upregulated in human melanocytes by UVB irradiation. J Am Acad Dermatol 42: 741–745.
Raj D, Liu T, Samadashwily G, Li F, Grossman D . (2008). Survivin repression by p53, Rb and E2F2 in normal human melanocytes. Carcinogenesis 29: 194–201.
Riley PA . (1997). Melanin. Int J Biochem Cell Biol 29: 1235–1239.
Serrano M, Lee H, Chin L, Cordon-Cardo C, Beach D, DePinho RA . (1996). Role of the INK4a locus in tumor suppression and cell mortality. Cell 85: 27–37.
Shapiro GI, Edwards CD, Ewen ME, Rollins BJ . (1998). p16INK4A participates in a G1 arrest checkpoint in response to DNA damage. Mol Cell Biol 18: 378–387.
Sharpless NE, DePinho RA . (1999). The INK4A/ARF locus and its two gene products. Curr Opin Genet Dev 9: 22–30.
Takahashi A, Ohtani N, Yamakoshi K, Iida S, Tahara H, Nakayama K et al. (2006). Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nat Cell Biol 8: 1291–1297.
Urabe K, Aroca P, Tsukamoto K, Mascagna D, Palumbo A, Prota G et al. (1994). The inherent cytotoxicity of melanin precursors: a revision. Biochim Biophys Acta 1221: 272–278.
Wang H-T, Choi B, Tang MS . (2010). Melanocytes are deficient in repair of oxidative DNA damage and UV-induced photoproducts. Proc Natl Acad Sci USA 107: 2180–2185.
Welm BE, Dijkgraaf GJ, Bledau AS, Welm AL, Werb Z . (2008). Lentiviral transduction of mammary stem cells for analysis of gene function during development and cancer. Cell Stem Cell 2: 90–102.
Wood SR, Berwick M, Ley RD, Walter RB, Setlow RB, Timmins GS . (2006). UV causation of melanoma in Xiphophorus is dominated by melanin photosensitized oxidant production. Proc Natl Acad Sci USA 103: 4111–4115.
Zyrek-Betts J, Micale M, Lineen A, Chaudhuri PK, Keil S, Xue J et al. (2008). Malignant blue nevus with lymph node metastases. J Cutan Pathol 35: 651–657.
Acknowledgements
This work was supported in part by NIH grants AR050102 and CA125761, the Department of Dermatology, and the Huntsman Cancer Foundation. We acknowledge use of the DNA/peptide and Flow Cytometry core facilities and a pilot project grant supported by P30 CA042014 awarded to Huntsman Cancer Institute. We thank Bryan Welm for providing the pEI2 plasmid and technical support for our lentiviral production and the MMHCC at the National Cancer Institute for providing B6.129-Cdkn2atm1Rdp mice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Jenkins, N., Liu, T., Cassidy, P. et al. The p16INK4A tumor suppressor regulates cellular oxidative stress. Oncogene 30, 265–274 (2011). https://doi.org/10.1038/onc.2010.419
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2010.419
Keywords
This article is cited by
-
Endosulfan affects embryonic development synergistically under elevated ambient temperature
Environmental Science and Pollution Research (2023)
-
Nacre Extract from Pearl Oyster Shell Prevents D-Galactose-Induced Brain and Skin Aging
Marine Biotechnology (2023)
-
KLF9-dependent ROS regulate melanoma progression in stage-specific manner
Oncogene (2019)
-
The interplay between p16 serine phosphorylation and arginine methylation determines its function in modulating cellular apoptosis and senescence
Scientific Reports (2017)
-
Transient postnatal overfeeding causes liver stress-induced premature senescence in adult mice
Scientific Reports (2017)