Abstract
Assembly of the preautophagosomal structure (PAS) is essential for autophagy initiation in yeast. Starvation-induced dephosphorylation of Atg13 is required for the formation of the Atg1–Atg13–Atg17–Atg29–Atg31 complex (Atg1 complex), a prerequisite for PAS assembly. However, molecular details underlying these events have not been established. Here we studied the interactions of yeast Atg13 with Atg1 and Atg17 by X-ray crystallography. Atg13 binds tandem microtubule interacting and transport domains in Atg1, using an elongated helix-loop-helix region. Atg13 also binds Atg17, using a short region, thereby bridging Atg1 and Atg17 and leading to Atg1-complex formation. Dephosphorylation of specific serines in Atg13 enhanced its interaction with not only Atg1 but also Atg17. These observations update the autophagy-initiation model as follows: upon starvation, dephosphorylated Atg13 binds both Atg1 and Atg17, and this promotes PAS assembly and autophagy progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mizushima, N. & Komatsu, M. Autophagy: renovation of cells and tissues. Cell 147, 728–741 (2011).
Mizushima, N., Yoshimori, T. & Ohsumi, Y. The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 27, 107–132 (2011).
Suzuki, K. & Ohsumi, Y. Current knowledge of the pre-autophagosomal structure (PAS). FEBS Lett. 584, 1280–1286 (2010).
Suzuki, K. et al. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 20, 5971–5981 (2001).
Noda, T. & Ohsumi, Y. Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J. Biol. Chem. 273, 3963–3966 (1998).
Kamada, Y. et al. Tor directly controls the Atg1 kinase complex to regulate autophagy. Mol. Cell. Biol. 30, 1049–1058 (2010).
Kamada, Y. et al. Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J. Cell Biol. 150, 1507–1513 (2000).
Kabeya, Y. et al. Characterization of the Atg17-Atg29-Atg31 complex specifically required for starvation-induced autophagy in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 389, 612–615 (2009).
Kawamata, T., Kamada, Y., Kabeya, Y., Sekito, T. & Ohsumi, Y. Organization of the pre-autophagosomal structure responsible for autophagosome formation. Mol. Biol. Cell 19, 2039–2050 (2008).
Cheong, H., Nair, U., Geng, J. & Klionsky, D.J. The Atg1 kinase complex is involved in the regulation of protein recruitment to initiate sequestering vesicle formation for nonspecific autophagy in Saccharomyces cerevisiae. Mol. Biol. Cell 19, 668–681 (2008).
Kraft, C. et al. Binding of the Atg1/ULK1 kinase to the ubiquitin-like protein Atg8 regulates autophagy. EMBO J. 31, 3691–3703 (2012).
Cheong, H. et al. Atg17 regulates the magnitude of the autophagic response. Mol. Biol. Cell 16, 3438–3453 (2005).
Kabeya, Y. et al. Atg17 functions in cooperation with Atg1 and Atg13 in yeast autophagy. Mol. Biol. Cell 16, 2544–2553 (2005).
Jao, C.C., Ragusa, M.J., Stanley, R.E. & Hurley, J.H.A. HORMA domain in Atg13 mediates PI 3-kinase recruitment in autophagy. Proc. Natl. Acad. Sci. USA 110, 5486–5491 (2013).
Chan, E.Y., Longatti, A., McKnight, N.C. & Tooze, S.A. Kinase-inactivated ULK proteins inhibit autophagy via their conserved C-terminal domains using an Atg13-independent mechanism. Mol. Cell. Biol. 29, 157–171 (2009).
Ragusa, M.J., Stanley, R.E. & Hurley, J.H. Architecture of the Atg17 complex as a scaffold for autophagosome biogenesis. Cell 151, 1501–1512 (2012).
Holm, L., Kaariainen, S., Rosenstrom, P. & Schenkel, A. Searching protein structure databases with DaliLite v.3. Bioinformatics 24, 2780–2781 (2008).
Hurley, J.H. & Yang, D. MIT domainia. Dev. Cell 14, 6–8 (2008).
Xiao, J. et al. Structural basis of Vta1 function in the multivesicular body sorting pathway. Dev. Cell 14, 37–49 (2008).
Matsuura, A., Tsukada, M., Wada, Y. & Ohsumi, Y. Apg1p, a novel protein kinase required for the autophagic process in Saccharomyces cerevisiae. Gene 192, 245–250 (1997).
Yeh, Y.Y., Wrasman, K. & Herman, P.K. Autophosphorylation within the Atg1 activation loop is required for both kinase activity and the induction of autophagy in Saccharomyces cerevisiae. Genetics 185, 871–882 (2010).
Noda, T., Matsuura, A., Wada, Y. & Ohsumi, Y. Novel system for monitoring autophagy in the yeast Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 210, 126–132 (1995).
Lynch-Day, M.A. & Klionsky, D.J. The Cvt pathway as a model for selective autophagy. FEBS Lett. 584, 1359–1366 (2010).
Mao, K. et al. Atg29 phosphorylation regulates coordination of the Atg17-Atg31-Atg29 complex with the Atg11 scaffold during autophagy initiation. Proc. Natl. Acad. Sci. USA 110, E2875–E2884 (2013).
Stephan, J.S., Yeh, Y.Y., Ramachandran, V., Deminoff, S.J. & Herman, P.K. The Tor and PKA signaling pathways independently target the Atg1/Atg13 protein kinase complex to control autophagy. Proc. Natl. Acad. Sci. USA 106, 17049–17054 (2009).
Soulard, A. et al. The rapamycin-sensitive phosphoproteome reveals that TOR controls protein kinase A toward some but not all substrates. Mol. Biol. Cell 21, 3475–3486 (2010).
Mizushima, N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr. Opin. Cell Biol. 22, 132–139 (2010).
Hara, T. et al. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J. Cell Biol. 181, 497–510 (2008).
Kobashigawa, Y., Kumeta, H., Ogura, K. & Inagaki, F. Attachment of an NMR-invisible solubility enhancement tag using a sortase-mediated protein ligation method. J. Biomol. NMR 43, 145–150 (2009).
Sikorski, R.S. & Hieter, P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19–27 (1989).
Otwinowski, Z. & Minor, W. Methods Enzymol. 276, 307–326 (1997).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Vagin, A. & Teplyakov, A. MOLREP: an automated program for molecular replacement. J. Appl. Crystallogr. 30, 1022–1025 (1997).
Winn, M.D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 (2011).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Brünger, A.T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 (1998).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Kaiser, C., Michaelis, S. & Mitchell, A. Methods in Yeast Genetics: a Cold Spring Harbor Laboratory Course Manual (Cold Spring Harbor Laboratory Press, 1994).
Janke, C. et al. A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast 21, 947–962 (2004).
Suzuki, K., Morimoto, M., Kondo, C. & Ohsumi, Y. Selective autophagy regulates insertional mutagenesis by the Ty1 retrotransposon in Saccharomyces cerevisiae. Dev. Cell 21, 358–365 (2011).
Tokunaga, M., Imamoto, N. & Sakata-Sogawa, K. Highly inclined thin illumination enables clear single-molecule imaging in cells. Nat. Methods 5, 159–161 (2008).
Acknowledgements
The synchrotron radiation experiments were performed at beamlines BL41XU at SPring8 and NW12A at Photon Factory, Japan. This work was supported in part by Japan Society for the Promotion of Sciences KAKENHI, grant nos. 24113725 and 25111004 (to N.N.N.), 2440279 (to Y.F.), 30114416 (to Y.O.) and 24770182 (to H.Y.), and by the Targeted Proteins Research Program from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to Y.O. and F.I.).
Author information
Authors and Affiliations
Contributions
Y.F. and N.N.N. performed structural studies; Y.F. performed biochemical studies; S.W.S., H.Y. and C.K.-K. performed yeast experiments; R.A. cloned the Atg homolog from K. marxianus; Y.K. and H.H. performed LC-MS/MS analysis; and Y.F., S.W.S., H.Y., F.I., Y.O. and N.N.N. analyzed data and wrote the manuscript. All authors discussed the results and commented on the manuscript. N.N.N. and Y.O. supervised the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Crystallographic study of the Atg1tMIT–Atg13MIM complex.
(a) Prediction of intrinsically disordered regions in S. cerevisiae Atg13 using the DISOPRED server1. The residues predicted to be disordered are colored red. (b) SDS-PAGE of in vitro pulldown assay between Atg13–GST and Atg1tMIT. As for Atg1tMIT bands, they were also detected by Western blotting using anti-Atg1 antibody. (c) CD spectra of Atg13MIM–GB1, GB1 alone, ubiquitin-associated domain (UBA) of p62 (p62UBA)–GB1 as a model of helix-rich domain2, and the flexible region (FR) of Arabidopsis thaliana (At) Atg3 (AtAtg3FR)–GB1 as a model of intrinsically disordered protein3,4. For CD spectroscopy, GB1, a small globular domain consisting of one α-helix and one four-stranded β-sheet, was used as a stabilization tag5. (d) Stereo view of the 2Fo-Fc electron density map of the Atg1tMIT–Atg13MIM complex countered at 1.5 σ level. The final model was shown with a stick model, in which carbon, nitrogen, oxygen and sulfur atoms were colored yellow, blue, red and green, respectively. This figure was prepared using COOT6. (e) Stereo view of the Cα-traces of the Atg1tMIT–Atg13MIM complex. (f) Superimposition of the Atg1–Atg13 complex (copy 2; colored gray) on that of copy 1 (colored red and blue). (g) Ribbon representation of the structure of Vta1tMIT–Vps60MIM complex (PDB ID 2LUH). MIT1 and MIT2 of Vta1 are colored blue and cyan, respectively, and Vps60 is colored salmon pink. Amino- and carboxy termini are denoted by N and C, respectively. Uncropped gel image for b is shown in Supplementary Fig. 6k.
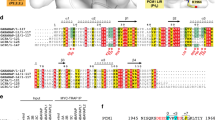
Supplementary Figure 2 The Atg1tMIT-Atg13MIM interaction is evolutionarily conserved.
(a,b) Amino-acid sequence alignment of Atg13 (a) and Atg1 (b) homologs using the ConSurf server7. Purple and cyan indicate high and low sequence conservation, respectively. The position of α-helices is indicated above the sequence. Surface representation of Atg1tMIT is inserted in b, whose coloring is the same as the sequence alignment. Atg13MIM bound to Atg1tMIT is shown by a ribbon model. (c) Sequence alignment between the Atg1/ULK1 binding regions of ScAtg13 and HsAtg13. Hydrophobic residues important for Atg1 binding are indicated with asterisks. The position of α-helices is indicated below the sequence. (d) SDS-PAGE of GST–HsAtg13 (458–517) co-purified with MmULK1 (834–1051). Uncropped gel image for d is shown in Supplementary Fig. 6l.
Supplementary Figure 3 Functional analysis of the Atg1tMIT-Atg13MIM interaction.
(a) CD spectra of Atg13MIM–GB1 (black) and Atg13MIM(F468A L472A)–GB1 (red). (b) SDS-PAGE of in vitro pulldown assay between Atg13MIM–GST and Atg1tMIT. (c) Western blots showing in vivo Atg1–Atg13 interaction analyzed by coimmunoprecipitation in the absence of Atg11 and Atg17. Samples are yeast total lysates (Input) prepared from ATG1–GFP atg11Δ atg17Δcells grown in nutrient-rich medium (Rapamycin, –) or treated with rapamycin for 1 h (Rapamycin, +) and immunoprecipitates isolated with anti-GFP magnetic beads (20xEluate). Asterisks indicate nonspecific bands. Atg13–P indicates phosphorylated forms of Atg13. (d) Western blots of yeast total lysates. Samples are yeast total lysates prepared from cells grown in nutrient-rich medium (Rapamycin, –) or treated with rapamycin for 1 h (Rapamycin, +). To detect slow-migrating phosphorylated forms of Atg1, SDS-PAGE was performed for prolonged period (~120 min) by using 10% polyacrylamide gel (10% gel). prApe1 and mApe1 indicate precursor and mature forms of Ape1, respectively. Uncropped gel images for b, c and d are shown in Supplementary Fig. 6m–o.
Supplementary Figure 4 Molecular characterization of the Atg1317BR-Atg17 interaction.
(a) SDS-PAGE of in vitro pulldown assay between GST–Atg13 and Atg17. (b) Stereo view of the electron density map of Atg1317BR bound to Atg17. The simulated annealing Fo-Fc difference Fourier map was calculated by omitting Atg1317BR, and is shown with black meshes countered at 3.0σ. Structural model of Atg13 and Atg17 is shown similarly with Fig. 3c, right. (c) Stereo view of the Fo-Fc difference Fourier map (countered at 3.0σ) at the Atg1317BR binding region of another Atg17 protomer. (d) CD spectra of Atg17(Wild-type), Atg17(D247A) and Atg17(D247A E250A) bound to Atg31(174–196). (e) SDS-PAGE of in vitro pulldown assay between Atg31(174–196)-bound Atg17 and GST–fused Atg1317BR. (f) ITC result obtained by titrating Atg1317BR into a solution of Atg17(D247A E250A). Uncropped gel images for a and e are shown in Supplementary Fig. 6p,q.
Supplementary Figure 5 Regulation of the interactions of Atg13 with Atg1 and Atg17.
(a) Western blots showing in vivo Atg1–Atg13 interaction analyzed by coimmunoprecipitation in different buffer conditions. Samples are yeast total lysates (Input) prepared from ATG1–GFP cells grown in nutrient-rich medium (Rapamycin, –) or treated with rapamycin for 1 h (Rapamycin, +) and immunoprecipitates isolated with anti-GFP magnetic beads (20xEluate) prepared from buffer conditions 1 and 2. Buffer condition 1 is the same as that used in other coimmunoprecipitation experiments performed in this manuscript. Buffer condition 2 is the same as that used in the recent paper by Kraft et al. Asterisks indicate nonspecific bands. Atg13–P indicates phosphorylated forms of Atg13. (b) Western blots using anti-HA antibody showing in vivo Atg1–Atg13 interaction analyzed by coimmunoprecipitation. Samples are yeast total lysates (Input) prepared from ATG1–GFP cells grown in nutrient-rich medium (Rapamycin, –) or treated with rapamycin for 1 h (Rapamycin, +) and immunoprecipitates isolated with anti-GFP magnetic beads (20xEluate). Atg13–2xHA–P indicates phosphorylated forms of Atg13–2xHA. λPPase indicates treatment of the immunoprecipitates with lambda protein phosphatase. (c) Western blots showing the effects of S429D mutation in Atg13 on the Atg1–Atg13 interaction analyzed by coimmunoprecipitation. Coimmunoprecipitation experiments and western blots were performed as in b. (d) Western blots showing the phosphorylation state of Atg13–2xHA probed with anti-HA antibody and phospho-specific anti-S428/9–P antibodies. Samples are immunoprecipitates of anti-HA immunoprecipitation prepared from cells grown in nutrient-rich medium (Rapamycin, –) or treated with rapamycin for 1 h (Rapamycin, +). (e) Immunoprecipitation and western blots performed as in d. λPPase indicates treatment of the immunoprecipitates with lambda protein phosphatase. Uncropped gel images are shown in Supplementary Fig. 6r–u.
Supplementary Figure 6 Uncropped images for western blots and SDS-PAGE gels.
Broken box marks the borders of the final cropped image.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Tables 1–3 and Supplementary Note (PDF 2173 kb)
Rights and permissions
About this article
Cite this article
Fujioka, Y., Suzuki, S., Yamamoto, H. et al. Structural basis of starvation-induced assembly of the autophagy initiation complex. Nat Struct Mol Biol 21, 513–521 (2014). https://doi.org/10.1038/nsmb.2822
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2822
This article is cited by
-
Decoding the function of Atg13 phosphorylation reveals a role of Atg11 in bulk autophagy initiation
EMBO Reports (2024)
-
Polphylipoprotein-induced autophagy mechanism with high performance in photodynamic therapy
Communications Biology (2023)
-
Effects of pH alterations on stress- and aging-induced protein phase separation
Cellular and Molecular Life Sciences (2022)
-
A separation that’s for the best: coming together at the PAS
Cell Research (2020)
-
Phase separation organizes the site of autophagosome formation
Nature (2020)