Abstract
The incidence of renal cell carcinoma (RCC) is increasing and outcomes remain poor. One-third of patients with localized disease will relapse, and 5-year survival for patients with metastatic disease is less than 10%. No molecular test is currently available to identify which patients who have undergone 'curative' surgery will relapse, and which patients will respond to targeted therapy. Some well characterized biochemical pathways, such as those associated with von Hippel–Lindau disease, are aberrantly regulated in RCC and are associated with histological subtype, but the understanding of these pathways contributes little to the clinical management of patients with RCC. Gene expression and sequencing studies have increased our understanding of the genetic basis of the disease but have failed to establish any unified classification to improve molecular stratification or to predict which patients are likely to relapse or respond to targeted therapy. Instead, they have served to highlight that RCC is heterogeneous at histological, morphological, and molecular levels, and that novel approaches are required to resolve the complexity of RCC prognostication and prediction of treatment response.
Key Points
-
Despite the prominence of molecular-targeted therapy in renal cell carcinoma (RCC), and the wealth of information on pathobiology, there are no molecular pathology tests to guide disease prognosis or predict treatment response
-
Both candidate and systematic approaches to selecting potential prognostic and predictive biomarkers in RCC from genetic and proteomic studies have failed
-
Generic and RCC-specific methodological, biological and pathological factors can account for the failure of previous studies to identify robust biomarkers in RCC
-
There is a high level of molecular and pathological heterogeneity in RCC, which has not been taken into account by existing genetic and proteomic approaches
-
More-detailed heterogeneity mapping is required to establish the importance of heterogeneity in prognosis and prediction of therapeutic response
-
Future predictive assays in RCC will need to be multiparametric, and incorporate novel, high-throughput, highly quantifiable technologies to account for the complexity of the pathway networks involved
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jemal, A., Siegel, R., Xu, J. & Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 60, 277–300 (2010).
CancerResearchUK. Kidney cancer statistics [online], (2011).
Linehan, W. M. et al. Molecular diagnosis and therapy of kidney cancer. Annu. Rev. Med. 61, 329–343 (2010).
Jayson, M. & Sanders, H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology 51, 203–205 (1998).
Hollingsworth, J. M., Miller, D. C., Daignault, S. & Hollenbeck, B. K. Rising incidence of small renal masses: a need to reassess treatment effect. J. Natl Cancer Inst. 98, 1331–1334 (2006).
Nguyen, M. M., Gill, I. S. & Ellison, L. M. The evolving presentation of renal carcinoma in the United States: trends from the Surveillance, Epidemiology, and End Results program. J. Urol. 176, 2397–2400 (2006).
Chow, W. H., Devesa, S. S., Warren, J. L. & Fraumeni, J. F. Jr. Rising incidence of renal cell cancer in the United States. JAMA 281, 1628–1631 (1999).
Touijer, K. et al. The expanding role of partial nephrectomy: a critical analysis of indications, results, and complications. Eur. Urol. 57, 214–222 (2010).
Heuer, R. et al. A critical analysis of the actual role of minimally invasive surgery and active surveillance for kidney cancer. Eur. Urol. 57, 223–232 (2010).
Chin, A. I., Lam, J. S., Figlin, R. A. & Belldegrun, A. S. Surveillance strategies for renal cell carcinoma patients following nephrectomy. Rev. Urol. 8, 1–7 (2006).
Levy, D. A., Slaton, J. W., Swanson, D. A. & Dinney, C. P. Stage specific guidelines for surveillance after radical nephrectomy for local renal cell carcinoma. J. Urol. 159, 1163–1167 (1998).
Chae, E. J., Kim, J. K., Kim, S. H., Bae, S. J. & Cho, K. S. Renal cell carcinoma: analysis of postoperative recurrence patterns. Radiology 234, 189–196 (2005).
Ljungberg, B. et al. Renal cell carcinoma guideline. Eur. Urol. 51, 1502–1510 (2007).
Cutress, M. L., Ratan, H. L., Williams, S. T. & O'Brien, M. F. Update on the management of T1 renal cortical tumours. BJU Int. 106, 1130–1136 (2010).
Vaishampayan, U. Metastatic renal cancer: a review of current and future treatment options. Am. J. Cancer 2, 201–210 (2003).
Flanigan, R. C. et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N. Engl. J. Med. 345, 1655–1659 (2001).
Di Lorenzo, G., Autorino, R. & Sternberg, C. N. Metastatic renal cell carcinoma: recent advances in the targeted therapy era. Eur. Urol. 56, 959–971 (2009).
McDermott, D. F. et al. The high-dose aldesleukin (HD IL-2) Select trial in patients with metastatic renal cell carcinoma (mRCC): preliminary assessment of clinical benefit. Presented at the ASCO 2010 Genitourinary Cancers Symposium.
Rini, B. I. & Flaherty, K. Clinical effect and future considerations for molecularly-targeted therapy in renal cell carcinoma. Urol. Oncol. 26, 543–549 (2008).
Swanton, C. et al. Predictive biomarker discovery through the parallel integration of clinical trial and functional genomics datasets. Genome Med. 2, 53 (2010).
De, P. & Leyland-Jones, B. Whither HER2-related therapeutics? J. Clin. Oncol. 28, 1091–1096 (2010).
Lord, C. J. & Ashworth, A. Biology-driven cancer drug development: back to the future. BMC Biol. 8, 38 (2010).
Wang, W. L. et al. Mechanisms of resistance to imatinib and sunitinib in gastrointestinal stromal tumor. Cancer Chemother. Pharmacol. 67 (Suppl. 1), S15–S24 (2011).
Rosner, I., Bratslavsky, G., Pinto, P. A. & Linehan, W. M. The clinical implications of the genetics of renal cell carcinoma. Urol. Oncol. 27, 131–136 (2009).
Renshaw, A. A. & Richie, J. P. Subtypes of renal cell carcinoma. Different onset and sites of metastatic disease. Am. J. Clin. Pathol. 111, 539–543 (1999).
Banumathy, G. & Cairns, P. Signaling pathways in renal cell carcinoma. Cancer Biol. Ther. 10, 658–664 (2010).
Verine, J. et al. Hereditary renal cancer syndromes: an update of a systematic review. Eur. Urol. doi:10.1016/j.eururo.2010.08.031.
Linehan, W. M., Srinivasan, R. & Schmidt, L. S. The genetic basis of kidney cancer: a metabolic disease. Nat. Rev. Urol. 7, 277–285 (2010).
Beroukhim, R. et al. Patterns of gene expression and copy-number alterations in von Hippel Lindau disease-associated and sporadic clear cell carcinoma of the kidney. Cancer Res. 69, 4674–4681 (2009).
Stolle, C. et al. Improved detection of germline mutations in the von Hippel-Lindau disease tumor suppressor gene. Hum. Mutat. 12, 417–423 (1998).
Cohen, H. T. & McGovern, F. J. Renal-cell carcinoma. N. Engl. J. Med. 353, 2477–2490 (2005).
Arsanious, A., Bjarnason, G. A. & Yousef, G. M. From bench to bedside: current and future applications of molecular profiling in renal cell carcinoma. Mol. Cancer 8, 20 (2009).
Boer, J. M. et al. Identification and classification of differentially expressed genes in renal cell carcinoma by expression profiling on a global human 31,500-element cDNA array. Genome Res. 11, 1861–1870 (2001).
Takahashi, M. et al. Gene expression profiling of clear cell renal cell carcinoma: gene identification and prognostic classification. Proc. Natl Acad. Sci. USA 98, 9754–9759 (2001).
Jones, J. et al. Gene signatures of progression and metastasis in renal cell cancer. Clin. Cancer Res. 11, 5730–5739 (2005).
Lenburg, M. E. et al. Previously unidentified changes in renal cell carcinoma gene expression identified by parametric analysis of microarray data. BMC Cancer 3, 31 (2003).
Yao, M. et al. Gene expression analysis of renal carcinoma: adipose differentiation-related protein as a potential diagnostic and prognostic biomarker for clear-cell renal carcinoma. J. Pathol. 205, 377–387 (2005).
Takahashi, M. et al. Molecular subclassification of kidney tumors and the discovery of new diagnostic markers. Oncogene 22, 6810–6818 (2003).
Sultmann, H. et al. Gene expression in kidney cancer is associated with cytogenetic abnormalities, metastasis formation, and patient survival. Clin. Cancer Res. 11, 646–655 (2005).
Young, A. N. et al. Expression profiling of renal epithelial neoplasms: a method for tumor classification and discovery of diagnostic molecular markers. Am. J. Pathol. 158, 1639–1651 (2001).
Higgins, J. P. Gene array studies in renal neoplasia. ScientificWorldJournal 6, 502–511 (2006).
Ficarra, V. et al. prognostic and therapeutic impact of the histopathologic definition of parenchymal epithelial renal tumors. Eur. Urol. doi:10.1016/j.eururo.2010.08.001.
Young, A. N. et al. Beta defensin-1, parvalbumin, and vimentin: a panel of diagnostic immunohistochemical markers for renal tumors derived from gene expression profiling studies using cDNA microarrays. Am. J. Surg. Pathol. 27, 199–205 (2003).
Pan, C. C., Chen, P. C. & Ho, D. M. The diagnostic utility of MOC31, BerEP4, RCC marker and CD10 in the classification of renal cell carcinoma and renal oncocytoma: an immunohistochemical analysis of 328 cases. Histopathology 45, 452–459 (2004).
Liu, L. et al. Immunohistochemical analysis of chromophobe renal cell carcinoma, renal oncocytoma, and clear cell carcinoma: an optimal and practical panel for differential diagnosis. Arch. Pathol. Lab. Med. 131, 1290–1297 (2007).
Allory, Y. et al. Profiling and classification tree applied to renal epithelial tumours. Histopathology 52, 158–166 (2008).
Bui, M. H. et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin. Cancer Res. 9, 802–811 (2003).
Perret, A. G., Clemencon, A., Li, G., Tostain, J. & Peoc'h, M. Differential expression of prognostic markers in histological subtypes of papillary renal cell carcinoma. BJU Int. 102, 183–187 (2008).
Moch, H. et al. High-throughput tissue microarray analysis to evaluate genes uncovered by cDNA microarray screening in renal cell carcinoma. Am. J. Pathol. 154, 981–986 (1999).
Liao, S. Y., Aurelio, O. N., Jan, K., Zavada, J. & Stanbridge, E. J. Identification of the MN/CA9 protein as a reliable diagnostic biomarker of clear cell carcinoma of the kidney. Cancer Res. 57, 2827–2831 (1997).
Liou, L. S. et al. Microarray gene expression profiling and analysis in renal cell carcinoma. BMC Urol. 4, 9 (2004).
Gieseg, M. A. et al. Expression profiling of human renal carcinomas with functional taxonomic analysis. BMC Bioinformatics 3, 26 (2002).
Brannon, A. R. et al. Molecular stratification of clear cell renal cell carcinoma by consensus clustering reveals distinct subtypes and survival patterns. Genes Cancer 1, 152–163 (2010).
Kosari, F. et al. Clear cell renal cell carcinoma: gene expression analyses identify a potential signature for tumor aggressiveness. Clin. Cancer Res. 11, 5128–5139 (2005).
Skubitz, K. M. & Skubitz, A. P. Differential gene expression in renal-cell cancer. J. Lab. Clin. Med. 140, 52–64 (2002).
Vasselli, J. R. et al. Predicting survival in patients with metastatic kidney cancer by gene-expression profiling in the primary tumor. Proc. Natl Acad. Sci. USA 100, 6958–6963 (2003).
Zhao, H. et al. Gene expression profiling predicts survival in conventional renal cell carcinoma. PLoS Med. 3, e13 (2006).
Eichelberg, C., Junker, K., Ljungberg, B. & Moch, H. Diagnostic and prognostic molecular markers for renal cell carcinoma: a critical appraisal of the current state of research and clinical applicability. Eur. Urol. 55, 851–863 (2009).
Vickers, M. M. & Heng, D. Y. Prognostic and predictive biomarkers in renal cell carcinoma. Target Oncol. 5, 85–94 (2010).
Hacker, K. E. & Rathmell, W. K. Emerging molecular classification in renal cell carcinoma: implications for drug development. Target Oncol. 5, 75–84 (2010).
Lam, J. S., Pantuck, A. J., Belldegrun, A. S. & Figlin, R. A. Protein expression profiles in renal cell carcinoma: staging, prognosis, and patient selection for clinical trials. Clin. Cancer Res. 13, 703s–708s (2007).
Yao, M. et al. VHL tumor suppressor gene alterations associated with good prognosis in sporadic clear-cell renal carcinoma. J. Natl Cancer Inst. 94, 1569–1575 (2002).
Schraml, P. et al. VHL mutations and their correlation with tumour cell proliferation, microvessel density, and patient prognosis in clear cell renal cell carcinoma. J. Pathol. 196, 186–193 (2002).
Baldewijns, M. M. et al. Different angiogenic potential in low and high grade sporadic clear cell renal cell carcinoma is not related to alterations in the von Hippel-Lindau gene. Cell Oncol. 31, 371–382 (2009).
Choueiri, T. K. et al. von Hippel-Lindau gene status and response to vascular endothelial growth factor targeted therapy for metastatic clear cell renal cell carcinoma. J. Urol. 180, 860–866 (2008).
Cho, D. et al. Potential histologic and molecular predictors of response to temsirolimus in patients with advanced renal cell carcinoma. Clin. Genitourin. Cancer 5, 379–385 (2007).
Rini, B. I. et al. Clinical response to therapy targeted at vascular endothelial growth factor in metastatic renal cell carcinoma: impact of patient characteristics and Von Hippel-Lindau gene status. BJU Int. 98, 756–762 (2006).
Kim, J. H. et al. Somatic VHL alteration and its impact on prognosis in patients with clear cell renal cell carcinoma. Oncol. Rep. 13, 859–864 (2005).
Klatte, T. et al. Hypoxia-inducible factor 1 alpha in clear cell renal cell carcinoma. Clin. Cancer Res. 13, 7388–7393 (2007).
Lidgren, A. et al. Hypoxia-inducible factor 1 alpha expression in renal cell carcinoma analyzed by tissue microarray. Eur. Urol. 50, 1272–1277 (2006).
Jacobsen, J., Rasmuson, T., Grankvist, K. & Ljungberg, B. Vascular endothelial growth factor as prognostic factor in renal cell carcinoma. J. Urol. 163, 343–347 (2000).
Na, X. et al. Overproduction of vascular endothelial growth factor related to von Hippel-Lindau tumor suppressor gene mutations and hypoxia-inducible factor-1 alpha expression in renal cell carcinomas. J. Urol. 170, 588–592 (2003).
Escudier, B. et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J. Clin. Oncol. 27, 3312–3318 (2009).
Rini, B. I. et al. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J. Clin. Oncol. 26, 3743–3748 (2008).
Porta, C. et al. Predictive value of baseline serum vascular endothelial growth factor and neutrophil gelatinase-associated lipocalin in advanced kidney cancer patients receiving sunitinib. Kidney Int. 77, 809–815 (2010).
Sabatino, M. et al. Serum vascular endothelial growth factor and fibronectin predict clinical response to high-dose interleukin-2 therapy. J. Clin. Oncol. 27, 2645–2652 (2009).
Escudier, B. J. et al. Update on AVOREN trial in metastatic renal cell carcinoma (mRCC): efficacy and safety in subgroups of patients (pts) and pharmacokinetic (PK) analysis [abstract 5025]. J. Clin. Oncol. 26 (Suppl.) (2008).
Lam, J. S., Leppert, J. T., Figlin, R. A. & Belldegrun, A. S. Role of molecular markers in the diagnosis and therapy of renal cell carcinoma. Urology 66, 1–9 (2005).
Leibovich, B. C. et al. Carbonic anhydrase IX is not an independent predictor of outcome for patients with clear cell renal cell carcinoma. J. Clin. Oncol. 25, 4757–4764 (2007).
Atkins, M. et al. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin. Cancer Res. 11, 3714–3721 (2005).
Pantuck, A. J. et al. Prognostic relevance of the mTOR pathway in renal cell carcinoma: implications for molecular patient selection for targeted therapy. Cancer 109, 2257–2267 (2007).
Klatte, T. et al. Molecular signatures of localized clear cell renal cell carcinoma to predict disease-free survival after nephrectomy. Cancer Epidemiol. Biomarkers Prev. 18, 894–900 (2009).
Kim, H. L. et al. Using protein expressions to predict survival in clear cell renal carcinoma. Clin. Cancer Res. 10, 5464–5471 (2004).
McShane, L. M. et al. REporting recommendations for tumor MARKer prognostic studies (REMARK). Nat. Clin. Pract. Urol. 2, 416–422 (2005).
Piccart-Gebhart, M. J. et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 353, 1659–1672 (2005).
Faratian, D., Clyde, R. G., Crawford, J. W. & Harrison, D. J. Systems pathology—taking molecular pathology into a new dimension. Nat.Rev. Clin. Oncol. 6, 455–464 (2009).
Tsao, M. S. et al. Erlotinib in lung cancer—molecular and clinical predictors of outcome. N. Engl. J. Med. 353, 133–144 (2005).
Faratian, D. et al. Systems biology reveals new strategies for personalizing cancer medicine and confirms the role of PTEN in resistance to trastuzumab. Cancer Res. 69, 6713–6720 (2009).
Barbie, D. A. et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature 462, 108–112 (2009).
Meylan, E. et al. Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma. Nature 462, 104–107 (2009).
Ebos, J. M. et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 15, 232–239 (2009).
Paez-Ribes, M. et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15, 220–231 (2009).
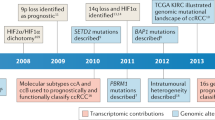
Kan, Z. et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature 466, 869–873 (2010).
Dalgliesh, G. L. et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature 463, 360–363 (2010).
Varela, I. et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature 469, 539–542 (2011).
Duns, G. et al. Histone methyltransferase gene SETD2 is a novel tumor suppressor gene in clear cell renal cell carcinoma. Cancer Res. 70, 4287–4291 (2010).
Dhillon, J. et al. Mucinous tubular and spindle cell carcinoma of the kidney with sarcomatoid change. Am. J. Surg. Pathol. 33, 44–49 (2009).
Yachida, S. et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 467, 1114–1117 (2010).
Huang, D. et al. Sunitinib acts primarily on tumor endothelium rather than tumor cells to inhibit the growth of renal cell carcinoma. Cancer Res. 70, 1053–1062 (2010).
Stewart, G. D. et al. The relevance of a hypoxic tumour microenvironment in prostate cancer. BJU Int. 105, 8–13 (2010).
Faratian, D., Langdon, S. P. & Harrison, D. J. How can systems pathology help us personalize cancer therapy? Discov. Med. 8, 81–86 (2009).
Sunitinib malate before and after surgery in treating patients with previously untreated metastatic kidney cancer [online], (2011).
Gonzalez-Angulo, A. M., Hennessy, B. T. & Mills, G. B. Future of personalized medicine in oncology: a systems biology approach. J. Clin. Oncol. 28, 2777–2783 (2010).
Malhotra, D. et al. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res. 38, 5718–5734 (2010).
Faratian, D., Bown, J. L., Smith, V. A., Langdon, S. P. & Harrison, D. J. Cancer systems biology. Methods Mol. Biol. 662, 245–263 (2010).
Faratian, D. et al. Phosphoprotein pathway profiling of ovarian carcinoma for the identification of potential new targets for therapy. Eur. J. Cancer doi:10.1016/j.ejca.2011.01.014.
Delahunt, B., Bethwaite, P. B., Thornton, A. & Ribas, J. L. Proliferation of renal cell carcinoma assessed by fixation-resistant polyclonal Ki-67 antibody labeling. Correlation with clinical outcome. Cancer 75, 2714–2719 (1995).
Rioux-Leclercq, N. et al. Value of immunohistochemical Ki-67 and p53 determinations as predictive factors of outcome in renal cell carcinoma. Urology 55, 501–505 (2000).
Visapaa, H. et al. Correlation of Ki-67 and gelsolin expression to clinical outcome in renal clear cell carcinoma. Urology 61, 845–850 (2003).
Bui, M. H. et al. Prognostic value of carbonic anhydrase IX and KI67 as predictors of survival for renal clear cell carcinoma. J. Urol. 171, 2461–2466 (2004).
Zigeuner, R., Ratschek, M., Rehak, P., Schips, L. & Langner, C. Value of p53 as a prognostic marker in histologic subtypes of renal cell carcinoma: a systematic analysis of primary and metastatic tumor tissue. Urology 63, 651–655 (2004).
Thompson, R. H. et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 66, 3381–3385 (2006).
Kallakury, B. V. et al. Increased expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma. Clin. Cancer Res. 7, 3113–3119 (2001).
Huang, D. et al. Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res. 70, 1063–1071 (2010).
Acknowledgements
The work of authors G. D. Stewart, F. C. O'Mahony, A. C. P. Riddick, D. J. Harrison and D. Faratian is funded by the Chief Scientist Office, grant number ETM37. The authors would like to thank SCOTRRCC coapplicants and collaborators for their useful discussions on some of the topics discussed in this Review. The authors would also like to acknowledge Dr. Gordon Mills for his helpful discussion developing the ideas behind biomarker properties and robustness. C. P. Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape, LLC-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Contributions
G. D. Stewart, F. C. O'Mahony and D. Faratian researched data for, and wrote, the article. All authors made a substantial contribution to discussions of content and were involved in the review and editing of the article before submission.
Corresponding author
Ethics declarations
Competing interests
G. D. Stewart declares an association with Pfizer (speakers bureau/honoraria).
T. Powles declares associations with Astra Zeneca (grant/research support), GlaxoSmithKline (grant/research support) and Pfizer (speakers bureau/honoraria and grant/research support).
Rights and permissions
About this article
Cite this article
Stewart, G., O'Mahony, F., Powles, T. et al. What can molecular pathology contribute to the management of renal cell carcinoma?. Nat Rev Urol 8, 255–265 (2011). https://doi.org/10.1038/nrurol.2011.43
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2011.43
This article is cited by
-
Exploring synthetic lethal network for the precision treatment of clear cell renal cell carcinoma
Scientific Reports (2022)
-
Identification of a differentiation-related prognostic nomogram based on single-cell RNA sequencing in clear cell renal cell carcinoma
Scientific Reports (2022)
-
RB1CC1 functions as a tumor-suppressing gene in renal cell carcinoma via suppression of PYK2 activity and disruption of TAZ-mediated PDL1 transcription activation
Cancer Immunology, Immunotherapy (2021)
-
The emerging role of the piRNA/piwi complex in cancer
Molecular Cancer (2019)
-
Overcoming intratumoural heterogeneity for reproducible molecular risk stratification: a case study in advanced kidney cancer
BMC Medicine (2017)