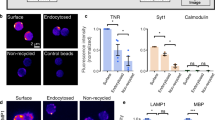
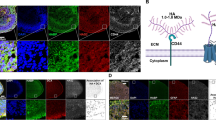
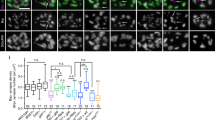
Key Points
-
In animals, organized groups of cells are surrounded by an extracellular matrix (ECM) of collagens, proteoglycans and glycoproteins. These molecules not only interact with each other, but they also activate signal transduction pathways, which coordinate cell proliferation, migration and differentiation.
-
In addition to its roles in development and regeneration in the nervous system, the ECM is involved in physiological processes in the adult brain, such as synaptic plasticity. This review focuses on this lesser-known function of ECM molecules in the adult nervous system.
-
Synaptic plasticity is the phenomenon by which the efficacy of synaptic transmission varies in an activity-dependent manner. A transient increase in synaptic efficacy is called short-term potentiation, whereas the persistent enhancement or reduction of synaptic strength of stimulated synapses are known as long-term potentiation (LTP) or long-term depression (LTD), respectively.
-
Because of the complexity and diversity of synaptic plasticity mechanisms, it is not difficult to imagine that more than one ECM molecule would affect synapse formation and synaptic modifications in the adult. To identify the best candidates, this review proposes a series of criteria that need to be fulfilled to show that an ECM molecule is required for synaptic plasticity.
-
ECM molecules that have been implicated in synaptic plasticity include laminins, reelin, heparin-binding growth-associated molecule (HB-GAM), neuronal activity-regulated pentraxin (Narp), tenascin-R, tenascin-C, and the chondroitin sulphate proteoglycans brevican and neurocan.
-
All of the ECM molecules that have been studied so far promote LTP in the CA1 region of the hippocampus, except for HB-GAM, which inhibits it. Short-term potentiation was typically not affected after manipulation of ECM molecules, except for reelin, which increased short-term potentiation.
-
These findings raise the question of how the effects of ECM molecules on synaptic plasticity are linked to developmental events. The sprouting of neurites that is stimulated by some of these molecules could promote the formation of new synapses, but it is equally conceivable that the barrier functions of certain ECM molecules are required for stabilization of nascent contacts.
Abstract
Interactions between cells and the extracellular matrix (ECM) have long been accepted to have pivotal roles in neural development and regeneration. Recent data also support the involvement of several ECM molecules in synaptic plasticity. Here, we review the present knowledge of the underlying mechanisms. These include interactions with cell surface receptors for ECM molecules coupled to the cytoskeleton and tyrosine kinase activities, and interactions with ion channels or neurotransmitter receptors. We hypothesize that ECM molecules derived from neurons and glia might also shape synaptic plasticity through regulation of organelle trafficking, and by imposing diffusion constraints for neurotransmitters and trophic factors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ruoslahti, E. Brain extracellular matrix. Glycobiology 6, 489–492 (1996).
Nicholson, C. & Sykova, E. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 21, 207–215 (1998).
Bruckner, G. et al. Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia 8, 183–200 (1993).
Celio, M. R., Spreafico, R., de Biasi, S. & Vitellaro-Zuccarello, L. Perineuronal nets: past and present. Trends Neurosci. 21, 510–515 (1998). This article provides a historical overview on visualization, composition and possible functions of perineuronal nets.
Yamaguchi, Y. Lecticans: organizers of the brain extracellular matrix. Cell. Mol. Life Sci. 57, 276–289 (2000).
Bruckner, G. et al. Extracellular matrix organization in various regions of rat brain grey matter. J. Neurocytol. 25, 333–346 (1996).
Matthews, R. T. et al. Aggrecan glycoforms contribute to the molecular heterogeneity of perineuronal nets. J. Neurosci. 22, 7536–7547 (2002).
Faissner, A. & Steindler, D. Boundaries and inhibitory molecules in developing neural tissues. Glia 13, 233–254 (1995).
Stichel, C. C. & Muller, H. W. The CNS lesion scar: new vistas on an old regeneration barrier. Cell Tissue Res. 294, 1–9 (1998).
Deller, T., Haas, C. A. & Frotscher, M. Reorganization of the rat fascia dentata after a unilateral entorhinal cortex lesion. Role of the extracellular matrix. Ann. NY Acad. Sci. 911, 207–220 (2000).
Jones, F. S. & Jones, P. L. The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev. Dyn. 218, 235–259 (2000).
Bandtlow, C. E. & Zimmermann, D. R. Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol. Rev. 80, 1267–1290 (2000). References 5, 11 and 12 describe interaction partners and functions of ECM molecules.
Geiger, B., Bershadsky, A., Pankov, R. & Yamada, K. M. Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nature Rev. Mol. Cell Biol. 2, 793–805 (2001).
Zucker, R. S. & Regehr, W. G. Short-term synaptic plasticity. Annu. Rev. Physiol. 64, 355–405 (2002).
Bliss, T. V. & Collingridge, G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–39 (1993).
Malenka, R. C. & Nicoll, R. A. Long-term potentiation — a decade of progress? Science 285, 1870–1874 (1999).
Lisman, J., Schulman, H. & Cline, H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nature Rev. Neurosci. 3, 175–190 (2002).
Ali, D. W. & Salter, M. W. NMDA receptor regulation by Src kinase signalling in excitatory synaptic transmission and plasticity. Curr. Opin. Neurobiol. 11, 336–342 (2001).
Kandel, E. R. The molecular biology of memory storage: a dialogue between genes and synapses. Science 294, 1030–1038 (2001).
Malinow, R. & Malenka, R. C. AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 25, 103–126 (2002). References 14–16 and 20 give nice introductions to the synaptic plasticity field.
Zakharenko, S. S., Zablow, L. & Siegelbaum, S. A. Visualization of changes in presynaptic function during long-term synaptic plasticity. Nature Neurosci. 4, 711–717 (2001).
Antonova, I. et al. Rapid increase in clusters of presynaptic proteins at onset of long-lasting potentiation. Science 294, 1547–1550 (2001).
Engert, F. & Bonhoeffer, T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399, 66–70 (1999).
Toni, N., Buchs, P. A., Nikonenko, I., Bron, C. R. & Muller, D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 402, 421–425 (1999).
Geinisman, Y. Structural synaptic modifications associated with hippocampal LTP and behavioral learning. Cereb. Cortex 10, 952–962 (2000).
Abraham, W. C. & Bear, M. F. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 19, 126–130 (1996).
Abraham, W. C. & Tate, W. P. Metaplasticity: a new vista across the field of synaptic plasticity. Prog. Neurobiol. 52, 303–323 (1997).
Wagner, J. J. & Alger, B. E. GABAergic and developmental influences on homosynaptic LTD and depotentiation in rat hippocampus. J. Neurosci. 15, 1577–1586 (1995).
Sanes, J. R. & Lichtman, J. W. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nature Rev. Neurosci. 2, 791–805 (2001).
Persohn, E., Pollerberg, G. E. & Schachner, M. Immunoelectron-microscopic localization of the 180 kD component of the neural cell adhesion molecule N-CAM in postsynaptic membranes. J. Comp. Neurol. 288, 92–100 (1989).
Kalb, R. G. & Hockfield, S. Molecular evidence for early activity-dependent development of hamster motor neurons. J. Neurosci. 8, 2350–2360 (1988).
Guimaraes, A., Zaremba, S. & Hockfield, S. Molecular and morphological changes in the cat lateral geniculate nucleus and visual cortex induced by visual deprivation are revealed by monoclonal antibodies Cat-304 and Cat-301. J. Neurosci. 10, 3014–3024 (1990).
Staubli, U., Vanderklish, P. & Lynch, G. An inhibitor of integrin receptors blocks long-term potentiation. Behav. Neural Biol. 53, 1–5 (1990).
Mayford, M., Barzilai, A., Keller, F., Schacher, S. & Kandel, E. R. Modulation of an NCAM-related adhesion molecule with long-term synaptic plasticity in Aplysia. Science 256, 638–644 (1992).
Lauri, S. E., Rauvala, H., Kaila, K. & Taira, T. Effect of heparin-binding growth-associated molecule (HB-GAM) on synaptic transmission and early LTP in rat hippocampal slices. Eur. J. Neurosci. 10, 188–194 (1998). This study describes the first evidence for the importance of the ECM component HB-GAM in hippocampal LTP.
Saghatelyan, A. K. et al. The extracellular matrix molecule tenascin-R and its HNK-1 carbohydrate modulate perisomatic inhibition and long-term potentiation in the CA1 region of the hippocampus. Eur. J. Neurosci. 12, 3331–3342 (2000). This work and reference 85 provide the first evidence for the importance of tenascin-R and its associated HNK-1 carbohydrate in regulation of perisomatic inhibition and LTP.
Nakagami, Y., Abe, K., Nishiyama, N. & Matsuki, N. Laminin degradation by plasmin regulates long-term potentiation. J. Neurosci. 20, 2003–2010 (2000).
Bukalo, O., Schachner, M. & Dityatev, A. Modification of extracellular matrix by enzymatic removal of chondroitin sulfate and by lack of tenascin-R differentially affects several forms of synaptic plasticity in the hippocampus. Neuroscience 104, 359–369 (2001). The study is the first to highlight the function of CSPGs in hippocampal LTP and LTD.
Evers, M. R. et al. Impairment of L-type Ca2+ channel-dependent forms of hippocampal synaptic plasticity in mice deficient in the extracellular matrix glycoprotein tenascin-C. J. Neurosci. 22, 7177–7194 (2002). The analysis of seven forms of long-term synaptic plasticity in the dentate gyrus and CA1 and CA3 areas of the hippocampus in the TN-C-deficient mutant disclosed an interplay between TN-C and Ca2+ channels.
Wright, J. W., Kramar, E. A., Meighan, S. E. & Harding, J. W. Extracellular matrix molecules, long-term potentiation, memory consolidation and the brain angiotensin system. Peptides 23, 221–246 (2002).
Sanes, J. R. & Lichtman, J. W. Can molecules explain long-term potentiation? Nature Neurosci. 2, 597–604 (1999).
Montanaro, F. et al. Laminin and α-dystroglycan mediate acetylcholine receptor aggregation via a MuSK-independent pathway. J. Neurosci. 18, 1250–1260 (1998).
Noakes, P. G., Gautam, M., Mudd, J., Sanes, J. R. & Merlie, J. P. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin β2. Nature 374, 258–262 (1995).
Patton, B. L. et al. Properly formed but improperly localized synaptic specializations in the absence of laminin α4. Nature Neurosci. 4, 597–604 (2001).
Marangi, P. A., Wieland, S. T. & Fuhrer, C. Laminin-1 redistributes postsynaptic proteins and requires rapsyn, tyrosine phosphorylation, and Src and Fyn to stably cluster acetylcholine receptors. J. Cell Biol. 157, 883–895 (2002).
Knight, D., Tolley, L. K., Kim, D. K., Lavidis, N. A. & Noakes, P. G. Functional analysis of neurotransmission at β2-laminin deficient terminals. J. Physiol. (Lond.) 546, 789–800 (2003).
Indyk, J. A., Chen, Z. L., Tsirka, S. E. & Strickland, S. Laminin chain expression suggests that laminin-10 is a major isoform in the mouse hippocampus and is degraded by the tissue plasminogen activator/plasmin protease cascade during excitotoxic injury. Neuroscience 116, 359–371 (2003).
Nayeem, N. et al. A potential role for the plasmin(ogen) system in the posttranslational cleavage of the neural cell adhesion molecule L1. J. Cell Sci. 112, 4739–4749 (1999).
Pinkstaff, J. K., Detterich, J., Lynch, G. & Gall, C. Integrin subunit gene expression is regionally differentiated in adult brain. J. Neurosci. 19, 1541–1556 (1999).
Schuster, T. et al. Immunoelectron microscopic localization of the neural recognition molecules L1, NCAM, and its isoform NCAM180, the NCAM-associated polysialic acid, β1 integrin and the extracellular matrix molecule tenascin-R in synapses of the adult rat hippocampus. J. Neurobiol. 49, 142–158 (2001).
Xiao, P., Bahr, B. A., Staubli, U., Vanderklish, P. W. & Lynch, G. Evidence that matrix recognition contributes to stabilization but not induction of LTP. Neuroreport 2, 461–464 (1991).
Staubli, U., Chun, D. & Lynch, G. Time-dependent reversal of long-term potentiation by an integrin antagonist. J. Neurosci. 18, 3460–3469 (1998).
Chun, D., Gall, C. M., Bi, X. & Lynch, G. Evidence that integrins contribute to multiple stages in the consolidation of long term potentiation in rat hippocampus. Neuroscience 105, 815–829 (2001).
Kramar, E. A., Bernard, J. A., Gall, C. M. & Lynch, G. α3 integrin receptors contribute to the consolidation of long-term potentiation. Neuroscience 110, 29–39 (2002).
Caviness, V. S. Jr & Rakic, P. Mechanisms of cortical development: a view from mutations in mice. Annu. Rev. Neurosci. 1, 297–326 (1978).
Miao, G. G. et al. Isolation of an allele of reeler by insertional mutagenesis. Proc. Natl Acad. Sci. USA 91, 11050–11054 (1994).
Tissir, F. & Goffinet, A. M. Reelin and brain development. Nature Rev. Neurosci. 4, 496–505 (2003).
Ishida, A., Shimazaki, K., Terashima, T. & Kawai, N. An electrophysiological and immunohistochemical study of the hippocampus of the reeler mutant mouse. Brain Res. 662, 60–68 (1994).
Weeber, E. J. et al. Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J. Biol. Chem. 277, 39944–39952 (2002). A combination of acute perturbation with analysis of mouse mutants deficient in Reln receptors uncovers some functions of Reln in synaptic plasticity.
Zhuo, M. et al. Role of tissue plasminogen activator receptor LRP in hippocampal long-term potentiation. J. Neurosci. 20, 542–549 (2000).
Verhey, K. J. et al. Cargo of kinesin identified as JIP scaffolding proteins and associated signaling molecules. J. Cell Biol. 152, 959–970 (2001).
Rauvala, H. & Peng, H. B. HB-GAM (heparin-binding growth-associated molecule) and heparin-type glycans in the development and plasticity of neuron-target contacts. Prog. Neurobiol. 52, 127–144 (1997).
Amet, L. E. et al. Enhanced hippocampal long-term potentiation in mice lacking heparin-binding growth-associated molecule. Mol. Cell. Neurosci. 17, 1014–1024 (2001).
Pavlov, I. et al. Role of heparin-binding growth-associated molecule (HB-GAM) in hippocampal LTP and spatial learning revealed by studies on overexpressing and knockout mice. Mol. Cell. Neurosci. 20, 330–342 (2002).
Lauri, S. E. et al. Regulatory role and molecular interactions of a cell-surface heparan sulfate proteoglycan (N-syndecan) in hippocampal long-term potentiation. J. Neurosci. 19, 1226–1235 (1999).
Kaksonen, M. et al. Syndecan-3-deficient mice exhibit enhanced LTP and impaired hippocampus-dependent memory. Mol. Cell. Neurosci. 21, 158–172 (2002).
Kinnunen, T. et al. Cortactin-Src kinase signaling pathway is involved in N-syndecan-dependent neurite outgrowth. J. Biol. Chem. 273, 10702–10708 (1998).
Naisbitt, S. et al. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron 23, 569–582 (1999).
Sheng, M. & Kim, M. J. Postsynaptic signaling and plasticity mechanisms. Science 298, 776–780 (2002).
Tsui, C. C. et al. Narp, a novel member of the pentraxin family, promotes neurite outgrowth and is dynamically regulated by neuronal activity. J. Neurosci. 16, 2463–2478 (1996).
O'Brien, R. J. et al. Synaptic clusterng of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron 23, 309–323 (1999). A novel mechanism for clustering of AMPA receptors by extracellularly secreted molecule is described.
O'Brien, R. J. et al. Synaptically targeted narp plays an essential role in the aggregation of AMPA receptors at excitatory synapses in cultured spinal neurons. J. Neurosci. 22, 4487–4498 (2002).
Mi, R. et al. Differing mechanisms for glutamate receptor aggregation on dendritic spines and shafts in cultured hippocampal neurons. J. Neurosci. 22, 7606–7616 (2002).
Hall, R. A. et al. Effects of heparin on the properties of solubilized and reconstituted rat brain AMPA receptors. Neurosci. Lett. 217, 179–183 (1996).
Sinnarajah, S. et al. Heparin modulates the single channel kinetics of reconstituted AMPA receptors from rat brain. Synapse 31, 203–209 (1999).
Faissner, A. & Schachner, M. in Neuroglia (eds Kettenmann, H. & Ransom, B. R.) 422–426 (Oxford Univ. Press, New York, 1995).
Nakic, M., Mitrovic, N., Sperk, G. & Schachner, M. Kainic acid activates transient expression of tenascin-C in the adult rat hippocampus. J. Neurosci. Res. 44, 355–362 (1996).
Nakic, M., Manahan-Vaughan, D., Reymann, K. G. & Schachner, M. Long-term potentiation in vivo increases rat hippocampal tenascin-C expression. J. Neurobiol. 37, 393–404 (1998).
Strekalova, T. et al. Fibronectin domains of extracellular matrix molecule tenascin-C modulate hippocampal learning and synaptic plasticity. Mol. Cell. Neurosci. 21, 173–187 (2002). A combined cell biological, electrophysiological and behavioural study on a distinct domain of TN-C in synaptic plasticity.
Dorries, U. et al. Distinct effects of recombinant tenascin-C domains on neuronal cell adhesion, growth cone guidance, and neuronal polarity. J. Neurosci. Res. 43, 420–438 (1996).
Srinivasan, J., Schachner, M. & Catterall, W. A. Interaction of voltage-gated sodium channels with the extracellular matrix molecules tenascin-C and tenascin-R. Proc. Natl Acad. Sci. USA 95, 15753–15757 (1998). References 79–81 highlight the interactions between ion channels and ECM molecules.
Weber, P. et al. Mice deficient for tenascin-R display alterations of the extracellular matrix and decreased axonal conduction velocities in the CNS. J. Neurosci. 19, 4245–4262 (1999).
Xiao, Z. C. et al. Tenascin-R is a functional modulator of sodium channel β-subunits. J. Biol. Chem. 274, 26511–26517 (1999).
Bruckner, G. et al. Postnatal development of perineuronal nets in wild-type mice and in a mutant deficient in tenascin-R. J. Comp. Neurol. 428, 616–629 (2000).
Saghatelyan, A. K. et al. Reduced perisomatic inhibition, increased excitatory transmission and impaired long-term potentiation in mice deficient for the extracellular matrix glycoprotein tenascin-R. Mol. Cell. Neurosci. 17, 226–240 (2001).
Schachner, M. & Martini, R. Glycans and the modulation of neural-recognition molecule function. Trends Neurosci. 18, 183–191 (1995).
Yamamoto, S. et al. Mice deficient in nervous system-specific carbohydrate epitope HNK-1 exhibit impaired synaptic plasticity and spatial learning. J. Biol. Chem. 277, 27227–27231 (2002).
Senn, C. et al. Mice deficient for the HNK-1 sulfotransferase show alterations in synaptic efficacy and spatial learning and memory. Mol. Cell. Neurosci. 20, 712–729 (2002).
Nikonenko, A., Schmidt, S., Skibo, G., Bruckner, G. & Schachner, M. Tenascin-R-deficient mice show structural alterations of symmetric perisomatic synapses in the CA1 region of the hippocampus. J. Comp. Neurol. 456, 338–349 (2003).
Saghatelyan, A. K. et al. Recognition molecule associated carbohydrate inhibits postsynaptic GABAB receptors: a mechanism for homeostatic regulation of GABA release in perisomatic synapses. Mol. Cell. Neurosci. (in the press).
Niederost, B. P., Zimmermann, D. R., Schwab, M. E. & Bandtlow, C. E. Bovine CNS myelin contains neurite growth-inhibitory activity associated with chondroitin sulfate proteoglycans. J. Neurosci. 19, 8979–8989 (1999).
Bradbury, E. J. et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416, 636–640 (2002).
Pizzorusso, T. et al. Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298, 1248–1251 (2002). The importance of CSPGs in regulating synaptic plasticity in the visual cortex in vivo is elegantly demonstrated in this paper.
Brakebusch, C. et al. Brevican-deficient mice display impaired hippocampal CA1 long-term potentiation but show no obvious deficits in learning and memory. Mol. Cell. Biol. 22, 7417–7427 (2002). This study shows that injection of brevican antibody and genetic ablation of brevican lead to abolishment of LTP.
Miura, R., Ethell, I. M. & Yamaguchi, Y. Carbohydrate-protein interactions between HNK-1-reactive sulfoglucuronyl glycolipids and the proteoglycan lectin domain mediate neuronal cell adhesion and neurite outgrowth. J. Neurochem. 76, 413–424 (2001).
Zhou, X. H. et al. Neurocan is dispensable for brain development. Mol. Cell. Biol. 21, 5970–5978 (2001).
Ngezahayo, A., Schachner, M. & Artola A. Synaptic activity modulates the induction of bidirectional synaptic changes in adult mouse hippocampus. J. Neurosci. 20, 2451–2458 (2000). A patch-clamp study that provides a framework for evaluation of depolarization thresholds for induction of LTP and LTD.
Fagiolini, M. & Hensch, T. K. Inhibitory threshold for critical-period activation in primary visual cortex. Nature 404, 183–186 (2000).
Rusakov, D. A. & Kullmann, D. M. Geometric and viscous components of the tortuosity of the extracellular space in the brain. Proc. Natl Acad. Sci. USA 95, 8975–8980 (1998).
Tian, M. et al. Laminin-α2 chain-like antigens in CNS dendritic spines. Brain Res. 764, 28–38 (1997).
Pappas, G. D., Kriho, V. & Pesold, C. Reelin in the extracellular matrix and dendritic spines of the cortex and hippocampus: a comparison between wild type and heterozygous reeler mice by immunoelectron microscopy. J. Neurocytol. 30, 413–425 (2001).
Wan, H. I. et al. Highwire regulates synaptic growth in Drosophila. Neuron 26, 313–329 (2000).
Sytnyk, V. et al. NCAM promotes accumulation of trans-Golgi network organelles at sites of neuron-to-neuron contacts. J. Cell Biol. 159, 649–661 (2002).
Son, Y. J. et al. The synaptic vesicle protein SV2 is complexed with an α5-containing laminin on the nerve terminal surface. J. Biol. Chem. 275, 451–460 (2000).
Brown, E. J. Integrin-associated proteins. Curr. Opin. Cell Biol. 14, 603–607 (2002).
Chavis, P. & Westbrook, G. Integrins mediate functional pre- and postsynaptic maturation at a hippocampal synapse. Nature 411, 317–321 (2001).
Beattie, E. C. et al. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nature Neurosci. 3, 1291–1300 (2000).
Passafaro, M., Piech, V. & Sheng, M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nature Neurosci. 4, 917–926 (2001).
Skeberdis, V. A., Lan, J., Zheng, X., Zukin, R. S. & Bennett, M. V. Insulin promotes rapid delivery of N-methyl-D-aspartate receptors to the cell surface by exocytosis. Proc. Natl Acad. Sci. USA 98, 3561–3566 (2001).
Wan, Q. et al. Recruitment of functional GABAA receptors to postsynaptic domains by insulin. Nature 388, 686–690 (1997).
Zhu, J., Qin, Y., Zhao, M., Van Aelst, L. & Malinow, R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell 110, 443–455 (2002). The study provides strong evidence for the involvement of small GTPases in receptor trafficking during LTP and LTD.
Sin, W. C., Haas, K., Ruthazer, E. S. & Cline, H. T. Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature 419, 475–480 (2002).
Ivins, J. K., Yurchenco, P. D. & Lander, A. D. Regulation of neurite outgrowth by integrin activation. J. Neurosci. 20, 6551–6560 (2000).
Bass, M. D. & Humphries, M. J. Cytoplasmic interactions of syndecan-4 orchestrate adhesion receptor and growth factor receptor signalling. Biochem. J. 368, 1–15 (2002).
Damsky, C. H. & Ilic, D. Integrin signaling: it's where the action is. Curr. Opin. Cell Biol. 14, 594–602 (2002).
Ziv, N. E. & Smith, S. J. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron 17, 91–102 (1996).
Shupliakov, O. et al. Impaired recycling of synaptic vesicles after acute perturbation of thepresynaptic actin cytoskeleton. Proc. Natl Acad. Sci. USA 99, 14476–14481 (2002).
Chang, S. & de Camilli, P. Glutamate regulates actin-based motility in axonal filopodia. Nature Neurosci. 4, 787–793 (2001).
Tanaka, H. et al. Molecular modification of N-cadherin in response to synaptic activity. Neuron 25, 93–107 (2000).
Scheiffele, P., Fan, J., Choih, J., Fetter, R. & Serafini, T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 101, 657–669 (2000).
Biederer, T. et al. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science 297, 1525–1531 (2002).
Contractor, A. et al. Trans-synaptic Eph receptor-ephrin signaling in hippocampal mossy fiber LTP. Science 296, 1864–1869 (2002).
Cole, G. J. & Glaser, L. A heparin-binding domain from N-CAM is involved in neural cell-substratum adhesion. J. Cell Biol. 102, 403–412 (1986).
Storms, S. D., Kim, A. C., Tran, B. H., Cole, G. J. & Murray, B. A. NCAM-mediated adhesion of transfected cells to agrin. Cell Adhes. Commun. 3, 497–509 (1996).
Kwan, C. P. et al. Probing fibroblast growth factor dimerization and role of heparin-like glycosaminoglycans in modulating dimerization and signaling. J. Biol. Chem. 276, 23421–23429 (2001).
Frame, M. C., Fincham, V. J., Carragher, N. O. & Wyke, J. A. v-Src's hold over actin and cell adhesions. Nature Rev. Mol. Cell Biol. 3, 233–245 (2002).
Fazeli, M. S., Breen, K., Errington, M. L. & Bliss, T. V. Increase in extracellular NCAM and amyloid precursor protein following induction of long-term potentiation in the dentate gyrus of anaesthetized rats. Neurosci. Lett. 169, 77–80 (1994).
Kalus, I., Schnegelsberg, B., Seidah, N. G., Kleene, R. & Schachner, M. The proprotein convertase PC5A and a metalloprotease are involved in the proteolytic processing of the neural adhesion molecule L1. J. Biol. Chem. 278, 10381–10388 (2003).
Wu, Y. P. et al. The tissue plasminogen activator (tPA)/plasmin extracellular proteolytic system regulates seizure-induced hippocampal mossy fiber outgrowth through a proteoglycan substrate. J. Cell Biol. 148, 1295–1304 (2000).
Baranes, D. et al. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron 21, 813–825 (1998).
Calabresi, P. et al. Tissue plasminogen activator controls multiple forms of synaptic plasticity and memory. Eur. J. Neurosci. 12, 1002–1012 (2000).
Komai, S. et al. Neuropsin regulates an early phase of Schaffer-collateral long-term potentiation in the murine hippocampus. Eur. J. Neurosci. 12, 1479–1486 (2000).
Hirata, A. et al. Abnormalities of synapses and neurons in the hippocampus of neuropsin-deficient mice. Mol. Cell. Neurosci. 17, 600–610 (2001).
D'Arcangelo, G. et al. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature 374, 719–723 (1995).
Shrive, A. K. et al. Three dimensional structure of human C-reactive protein. Nat. Struct. Biol. 3, 346–354 (1996).
Acknowledgements
We greatly appreciate helpful discussions with H. Beck, V. Bolshakov, G. Bruckner, P. Giese, M. Kneussel, D. Kullmann, A. Luthi and contributions from all members of our laboratory cited in the references. This work was supported by grants from the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Glossary
- PROTEOGLYCANS
-
The proteoglycans have a much higher ratio of polysaccharide to protein than do collagens, fibronectin, and glycoproteins in the extracellular matrix. The polysaccharide chains in proteoglycans are long repeating linear polymers of specific disaccharides called glycosaminoglycans. Usually one sugar is a uronic acid and the other is either N-acetylglucosamine or N-acetylgalactosamine.
- SPINES
-
Specialized regions of the dendrite that receive synaptic inputs from other neurons.
- ACTIVE ZONE
-
A portion of the presynaptic membrane that faces the postsynaptic density across the synaptic cleft. It constitutes the site of synaptic vesicle clustering, docking and transmitter release.
- METABOTROPIC
-
A term that describes a receptor that is associated with G proteins and exerts its effects through enzyme activation.
- SCHAFFER COLLATERALS
-
Axons of the CA3 pyramidal cells of the hippocampus that form synapses with the apical dendrites of CA1 neurons.
- CHONDROITIN SULPHATE PROTEOGLYCANS
-
Important components of the extracellular matrix and connective tissue. These proteins contain hydrophilic, negatively charged polymers of glucuronic acid and sulphated N-acetyl glucosamine residues.
- CONTEXTUAL FEAR CONDITIONING
-
Hippocampus-dependent learning task in which animals associate a specific location and its surroundings with an electric shock.
- ELEVATED PLUS-MAZE
-
In this experiment, animals are placed in the centre of an elevated four arm plus-like maze, in which two arms are closed and two arms are open. So, the animal can fall down from the open arms. Avoidance of being in open arms is taken as a measure of fearful behaviour.
- IMMEDIATE EARLY GENES
-
Genes that are induced rapidly and transiently. Many immediate-early genes, such as fos, control the transcription of other genes, and thereby regulate expression of sets of proteins.
- DOMINANT-NEGATIVE
-
A mutant molecule that binds to an interaction partner of the normal molecule and thereby blocks the functional complex.
- STEP-DOWN PASSIVE AVOIDANCE TASK
-
A behavioural experiment, in which an animal learns to associate stepping down from a raised platform with an aversive stimulus, such as electric shock. The name of the task derives from the fact that the animal learns to passively stay at the platform to avoid the stimulus.
- WATER-MAZE TASK
-
A learning task in which an animal is placed in a pool filled with opaque water and has to learn to escape to a hidden platform that is placed at a constant position. The animal must learn to use distal cues, and the spatial relationship between them and the platform.
- PATCH CLAMP
-
Technique whereby a small electrode tip is sealed onto a patch of cell membrane, making it possible to record the flow of current through individual ion channels or pores within the patch. Disruption of the patch membrane provides the possibility to record currents from the whole cell.
- G-PROTEIN-COUPLED INWARDLY RECTIFYING K+ CHANNELS
-
K+ channels that are regulated by neurotransmitters and hormones through G-protein-coupled receptors. They are called inward rectifiers because current flows through them more easily into than out of cells.
- PEPTIDOMIMETICS
-
Peptides mimicking other molecules — for instance carbohydrates — in their ability to bind other molecules.
- OCULAR DOMINANCE
-
In the mature primary visual cortex of mammals, most neurons respond predominantly to visual inputs from one eye or the other. This phenomenon is called ocular dominance. Cells that respond to a given eye are arranged in stripes — the ocular dominance columns — that alternate with stripes of neurons that respond to the other eye.
- SYNAPTOSOMES
-
A preparation of presynaptic terminals and postsynaptic membranes, isolated after subcellular fractionation. These structures can take up, store and release neurotransmitters and could contain postsynaptic densities.
- MOSSY FIBRES
-
Axons of dentate gyrus granule cells, which constitute the main excitatory input to CA3 pyramidal cells in the hippocampus.
Rights and permissions
About this article
Cite this article
Dityatev, A., Schachner, M. Extracellular matrix molecules and synaptic plasticity. Nat Rev Neurosci 4, 456–468 (2003). https://doi.org/10.1038/nrn1115
Issue Date:
DOI: https://doi.org/10.1038/nrn1115
This article is cited by
-
Collagen in the central nervous system: contributions to neurodegeneration and promise as a therapeutic target
Molecular Neurodegeneration (2024)
-
Circulating myeloid-derived MMP8 in stress susceptibility and depression
Nature (2024)
-
Timing is key for behavioural benefits of psychedelics
Nature (2023)
-
Extracellular Matrix in Neural Plasticity and Regeneration
Cellular and Molecular Neurobiology (2022)
-
Perineuronal nets stabilize the grid cell network
Nature Communications (2021)