Key Points
-
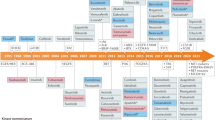
Small-molecule kinase inhibitors are being intensively pursued as new anticancer therapeutics. To date, approximately 80 inhibitors have been advanced to some stage of clinical evaluation.
-
Understanding the structural basis of kinase inhibitor selectivity is crucial to the ultimate goal of developing selective inhibitors to target every member of the kinome. Most currently known kinase inhibitors target the ATP binding site with the kinase activation loop in the active (type 1) or inactive (type 2) conformation.
-
New kinase inhibitors are primarily developed with a combination of methods, including high-throughput screening using biochemical or cellular assays, analogue synthesis, structure-guided design and fragment-based assembly strategies.
-
The repertoire of kinases targeted by a given inhibitor can be determined by profiling its activity in binding and enzymatic assays against extensive panels of recombinant kinases, by profiling activity in cellular assays and by affinity approaches integrated with detection by mass spectrometry.
-
Kinase inhibitor resistance resulting from selection for mutant alleles or upregulation of alternative signalling pathways is a recurrent theme in the clinic. Strategies for developing multiple inhibitors targeting different kinase sites and for discovering synergistic inhibitor combinations are urgently needed.
Abstract
Deregulation of kinase activity has emerged as a major mechanism by which cancer cells evade normal physiological constraints on growth and survival. To date, 11 kinase inhibitors have received US Food and Drug Administration approval as cancer treatments, and there are considerable efforts to develop selective small molecule inhibitors for a host of other kinases that are implicated in cancer and other diseases. Herein we discuss the current challenges in the field, such as designing selective inhibitors and developing strategies to overcome resistance mutations. This Review provides a broad overview of some of the approaches currently used to discover and characterize new kinase inhibitors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cohen, P. Protein kinases — the major drug targets of the twenty-first century? Nature Rev. Drug Discov. 1, 309–315 (2002).
Daley, G. Q., Van Etten, R. A. & Baltimore, D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science 247, 824–830 (1990).
Druker, B. J. et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl. J. Med. 355, 2408–2417 (2006).
Davies, S. P., Reddy, H., Caivano, M. & Cohen, P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351, 95–105 (2000).
Lombardo, L. J. et al. Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J. Med. Chem. 47, 6658–6661 (2004). This paper documents the discovery of dasatinib using a medicinal chemistry approach.
Shah, N. P. et al. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science 305, 399–401 (2004). This study showed that dasatinib inhibits many imatinib-resistant BCR–ABL1 mutants.
O'Brien, S. G. et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N. Engl. J. Med. 348, 994–1004 (2003).
Weinstein, I. B. et al. Disorders in cell circuitry associated with multistage carcinogenesis: exploitable targets for cancer prevention and therapy. Clin. Cancer Res. 3, 2696–2702 (1997).
Weinstein, I. B. & Joe, A. K. Mechanisms of disease: Oncogene addiction — a rationale for molecular targeting in cancer therapy. Nature Clin. Pract. Oncol. 3, 448–457 (2006).
Futreal, P. A. et al. A census of human cancer genes. Nature Rev. Cancer 4, 177–183 (2004). This article features an impressive compilation of genes known to be mutated in cancer.
Forbes, S. et al. Cosmic 2005. Br. J. Cancer 94, 318–322 (2006).
Samuels, Y. et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 304, 554 (2004).
Bachman, K. E. et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol. Ther. 3, 772–775 (2004).
Samuels, Y. et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell 7, 561–573 (2005).
Garnett, M. J. & Marais, R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell 6, 313–319 (2004).
Davies, H. et al. Mutations of the BRAF gene in human cancer. Nature 417, 949–954 (2002).
Morgan, K. J. & Gilliland, D. G. A role for JAK2 mutations in myeloproliferative diseases. Annu. Rev. Med. 59, 213–222 (2008).
Mosse, Y. P. et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 455, 930–935 (2008).
Kaelin, W. G. Jr. The concept of synthetic lethality in the context of anticancer therapy. Nature Rev. Cancer 5, 689–698 (2005).
Wang, J. Y., Wilcoxen, K. M., Nomoto, K. & Wu, S. Recent advances of MEK inhibitors and their clinical progress. Curr. Top. Med. Chem. 7, 1364–1378 (2007).
Faivre, S., Kroemer, G. & Raymond, E. Current development of mTOR inhibitors as anticancer agents. Nature Rev. Drug Discov. 5, 671–688 (2006).
Hu, Y. et al. 90-kDa ribosomal S6 kinase is a direct target for the nuclear fibroblast growth factor receptor 1 (FGFR1): role in FGFR1 signaling. J. Biol. Chem. 279, 29325–29335 (2004).
Malumbres, M. & Barbacid, M. Cell cycle kinases in cancer. Curr. Opin. Genet. Dev. 17, 60–65 (2007). A comprehensive review of the cell cycle kinases currently being developed as targets for novel inhibitors.
Geiger, T. R. & Peeper, D. S. Critical role for TrkB kinase function in anoikis suppression, tumorigenesis, and metastasis. Cancer Res. 67, 6221–6229 (2007).
Kerbel, R. S. Tumor angiogenesis. N. Engl. J. Med. 358, 2039–2049 (2008).
Christofk, H. R. et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452, 230–233 (2008).
Greenman, C. et al. Patterns of somatic mutation in human cancer genomes. Nature 446, 153–158 (2007).
Jia, S. et al. Essential roles of PI(3)K-p110β in cell growth, metabolism and tumorigenesis. Nature 454, 776–779 (2008).
Wee, S. et al. PTEN-deficient cancers depend on PIK3CB. Proc. Natl Acad. Sci. USA 105, 13057–13062 (2008).
Johnson, L. N., Lowe, E. D., Noble, M. E. & Owen, D. J. The Eleventh Datta Lecture. The structural basis for substrate recognition and control by protein kinases. FEBS Lett. 430, 1–11 (1998).
Manning, G., Whyte, D. B., Martinez, R., Hunter, T. & Sudarsanam, S. The protein kinase complement of the human genome. Science 298, 1912–1934 (2002).
Traxler, P. & Furet, P. Strategies toward the design of novel and selective protein tyrosine kinase inhibitors. Pharmacol. Ther. 82, 195–206 (1999).
Liu, Y. & Gray, N. S. Rational design of inhibitors that bind to inactive kinase conformations. Nature Chem. Biol. 2, 358–364 (2006).
Hennequin, L. F. et al. N.-(5-chloro-1,3-benzodioxol-4-yl)-7-[2-(4-methylpiperazin-1-yl)ethoxy]-5-(tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine, a novel, highly selective, orally available, dual-specific c-Src/Abl kinase inhibitor. J. Med. Chem. 49, 6465–6488 (2006).
Manley, P. W., Cowan-Jacob, S. W. & Mestan, J. Advances in the structural biology, design and clinical development of Bcr-Abl kinase inhibitors for the treatment of chronic myeloid leukaemia. Biochim. Biophys. Acta 1754, 3–13 (2005).
Wan, P. T. et al. Mechanism of activation of the RAF–ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116, 855–867 (2004).
Knight, Z. A. et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell 125, 733–747 (2006). An impressive illustration of the integration of chemical and biological approaches to discover and characterize isoform-selective PI3K inhibitors.
Ohren, J. F. et al. Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nature Struct. Mol. Biol. 11, 1192–1197 (2004).
Adrian, F. J. et al. Allosteric inhibitors of Bcr-abl-dependent cell proliferation. Nature Chem. Biol. 2, 95–102 (2006).
Barnett, S. F. et al. Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors. Biochem. J. 385, 399–408 (2005).
Lindsley, C. W. et al. Allosteric Akt (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorg. Med. Chem. Lett. 15, 761–764 (2005).
McIntyre, K. W. et al. A highly selective inhibitor of IκB kinase, BMS-345541, blocks both joint inflammation and destruction in collagen-induced arthritis in mice. Arthritis Rheum. 48, 2652–2659 (2003).
Grimsby, J. et al. Allosteric activators of glucokinase: potential role in diabetes therapy. Science 301, 370–373 (2003).
Guertin, K. R. & Grimsby, J. Small molecule glucokinase activators as glucose lowering agents: a new paradigm for diabetes therapy. Curr. Med. Chem. 13, 1839–1843 (2006).
Sullivan, J. E. et al. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 353, 33–36 (1994).
Sanders, M. J. et al. Defining the mechanism of activation of AMP-activated protein kinase by the small molecule A-769662, a member of the thienopyridone family. J. Biol. Chem. 282, 32539–32548 (2007).
Cohen, M. S., Zhang, C., Shokat, K. M. & Taunton, J. Structural bioinformatics-based design of selective, irreversible kinase inhibitors. Science 308, 1318–1321 (2005). This study is an instructive example of how a selective irreversible inhibitor of RSK was designed.
Kwak, E. L. et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc. Natl Acad. Sci. USA 102, 7665–7670 (2005).
Rabindran, S. K. et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 64, 3958–3965 (2004).
Kobayashi, S. et al. An alternative inhibitor overcomes resistance caused by a mutation of the epidermal growth factor receptor. Cancer Res. 65, 7096–7101 (2005).
Fry, D. W. et al. A specific inhibitor of the epidermal growth factor receptor tyrosine kinase. Science 265, 1093–1095 (1994).
Heymach, J. V., Nilsson, M., Blumenschein, G., Papadimitrakopoulou, V. & Herbst, R. Epidermal growth factor receptor inhibitors in development for the treatment of non-small cell lung cancer. Clin. Cancer Res. 12, 4441s–4445s (2006).
Felip, E., Santarpia, M. & Rosell, R. Emerging drugs for non-small-cell lung cancer. Expert Opin. Emerg. Drugs 12, 449–460 (2007).
Wissner, A. et al. Dual irreversible kinase inhibitors: quinazoline-based inhibitors incorporating two independent reactive centers with each targeting different cysteine residues in the kinase domains of EGFR and VEGFR-2. Bioorg. Med. Chem. 15, 3635–3648 (2007).
Pan, Z. et al. Discovery of selective irreversible inhibitors for Bruton's tyrosine kinase. ChemMedChem 2, 58–61 (2007).
Cohen, M. S., Hadjivassiliou, H. & Taunton, J. A clickable inhibitor reveals context-dependent autoactivation of p90 RSK. Nature Chem. Biol. 3, 156–160 (2007).
Schirmer, A., Kennedy, J., Murli, S., Reid, R. & Santi, D. V. Targeted covalent inactivation of protein kinases by resorcylic acid lactone polyketides. Proc. Natl Acad. Sci. USA 103, 4234–4239 (2006).
Li, B., Liu, Y., Uno, T. & Gray, N. Creating chemical diversity to target protein kinases. Comb. Chem. High Throughput Screen 7, 453–472 (2004).
Kraker, A. J. et al. Biochemical and cellular effects of c-Src kinase-selective pyrido[2,3-d]pyrimidine tyrosine kinase inhibitors. Biochem. Pharmacol. 60, 885–898 (2000).
Mohammadi, M. et al. Crystal structure of an angiogenesis inhibitor bound to the FGF receptor tyrosine kinase domain. EMBO J. 17, 5896–5904 (1998).
Lenart, P. et al. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr. Biol. 17, 304–315 (2007).
Sapkota, G. P. et al. BI-D1870 is a specific inhibitor of the p90 RSK (ribosomal S6 kinase) isoforms in vitro and in vivo. Biochem. J. 401, 29–38 (2007).
Scotlandi, K. et al. Antitumor activity of the insulin-like growth factor-I receptor kinase inhibitor NVP-AEW541 in musculoskeletal tumors. Cancer Res. 65, 3868–3876 (2005).
Okram, B. et al. A general strategy for creating “inactive-conformation” abl inhibitors. Chem. Biol. 13, 779–786 (2006).
Dubinina, G. G. et al. In silico design of protein kinase inhibitors: successes and failures. Anticancer Agents Med. Chem. 7, 171–188 (2007).
Gill, A. New lead generation strategies for protein kinase inhibitors — fragment based screening approaches. Mini Rev. Med. Chem. 4, 301–311 (2004).
Wyatt, P. G. et al. Identification of N-(4-piperidinyl)-4-(2,6-dichlorobenzoylamino)-1H-pyrazole-3-carboxamide (AT7519), a novel cyclin dependent kinase inhibitor using fragment-based X-ray crystallography and structure based drug design. J. Med. Chem. 51, 4986–4999 (2008).
Bain, J. et al. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 408, 297–315 (2007).
Venter, J. C. et al. The sequence of the human genome. Science 291, 1304–1351 (2001).
Carter, T. A. et al. Inhibition of drug-resistant mutants of, ABL, KIT, and EGF receptor kinases. Proc. Natl Acad. Sci. USA 102, 11011–11016 (2005).
Fabian, M. A. et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nature Biotechnol. 23, 329–336 (2005).
Harrington, E. A. et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nature Med. 10, 262–267 (2004).
Young, M. A. et al. Structure of the kinase domain of an imatinib-resistant Abl mutant in complex with the Aurora kinase inhibitor VX-680. Cancer Res. 66, 1007–1014 (2006).
Fedorov, O. et al. A systematic interaction map of validated kinase inhibitors with Ser/Thr kinases. Proc. Natl Acad. Sci. USA 104, 20523–20528 (2007).
Warmuth, M., Kim, S., Gu, X. J., Xia, G. & Adrian, F. Ba/F3 cells and their use in kinase drug discovery. Curr. Opin. Oncol. 19, 55–60 (2007).
Melnick, J. S. et al. An efficient rapid system for profiling the cellular activities of molecular libraries. Proc. Natl Acad. Sci. USA 103, 3153–3158 (2006).
Godl, K. et al. An efficient proteomics method to identify the cellular targets of protein kinase inhibitors. Proc. Natl Acad. Sci. USA 100, 15434–15439 (2003).
Bantscheff, M. et al. Quantitative chemical proteomics reveals mechanisms of action of clinical ABL kinase inhibitors. Nature Biotechnol. 25, 1035–1044 (2007).
Peters, E. C. & Gray, N. S. Chemical proteomics identifies unanticipated targets of clinical kinase inhibitors. ACS Chem. Biol. 2, 661–664 (2007).
le Coutre, P. et al. Induction of resistance to the Abelson inhibitor STI571 in human leukemic cells through gene amplification. Blood 95, 1758–1766 (2000).
Engelman, J. A. et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316, 1039–1043 (2007). This article describes upregulation of an alternative pathway as a mechanism of resistance to kinase inhibitors.
Chu, S. et al. Detection of BCR–ABL kinase mutations in CD34+ cells from chronic myelogenous leukemia patients in complete cytogenetic remission on imatinib mesylate treatment. Blood 105, 2093–2098 (2005).
Hughes, T. & Branford, S. Molecular monitoring of BCR–ABL as a guide to clinical management in chronic myeloid leukaemia. Blood Rev. 20, 29–41 (2006).
Roumiantsev, S. et al. Clinical resistance to the kinase inhibitor STI-571 in chronic myeloid leukemia by mutation of Tyr-253 in the Abl kinase domain P-loop. Proc. Natl Acad. Sci. USA 99, 10700–10705 (2002).
Fletcher, J. A. & Rubin, B. P. KIT mutations in GIST. Curr. Opin. Genet. Dev. 17, 3–7 (2007).
Cools, J. et al. Prediction of resistance to small molecule FLT3 inhibitors: implications for molecularly targeted therapy of acute leukemia. Cancer Res. 64, 6385–6389 (2004).
Graham, S. M. et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood 99, 319–325 (2002).
Copland, M. et al. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood 107, 4532–4539 (2006).
Pao, W. et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2, e73 (2005).
Blencke, S. et al. Characterization of a conserved structural determinant controlling protein kinase sensitivity to selective inhibitors. Chem. Biol. 11, 691–701 (2004). This article provides an impressive illustration of the general importance of the gatekeeper position.
Gumireddy, K. et al. A non-ATP-competitive inhibitor of BCR–ABL overrides imatinib resistance. Proc. Natl Acad. Sci. USA 102, 1992–1997 (2005).
Gumireddy, K. et al. ON01910, a non-ATP-competitive small molecule inhibitor of Plk1, is a potent anticancer agent. Cancer Cell 7, 275–286 (2005).
Gorre, M. E., Ellwood-Yen, K., Chiosis, G., Rosen, N. & Sawyers, C. L. BCR–ABL point mutants isolated from patients with imatinib mesylate-resistant chronic myeloid leukemia remain sensitive to inhibitors of the BCR–ABL chaperone heat shock protein 90. Blood 100, 3041–3044 (2002).
Copland, M. et al. BMS-214662 potently induces apoptosis of chronic myeloid leukemia stem and progenitor cells and synergises with tyrosine kinase inhibitors. Blood 111, 2843–2853 (2008).
Hamby, J. M. et al. Structure-activity relationships for a novel series of pyrido[2,3-d]pyrimidine tyrosine kinase inhibitors. J. Med. Chem. 40, 2296–2303 (1997).
Kothe, M. et al. Selectivity-determining residues in Plk1. Chem. Biol. Drug Des. 70, 540–546 (2007).
Weisberg, E. et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr–Abl. Cancer Cell 7, 129–141 (2005).
Shah, N. P. et al. Dasatinib (BMS-354825) inhibits KITD816V, an imatinib-resistant activating mutation that triggers neoplastic growth in most patients with systemic mastocytosis. Blood 108, 286–291 (2006).
Karaman, M. W. et al. A quantitative analysis of kinase inhibitor selectivity. Nature Biotechnol. 26, 127–132 (2008). This article features an impressive profiling of inhibitors against a large panel of kinases.
Vajpai, N. et al. Solution conformations and dynamics of ABL kinase-inhibitor complexes determined by NMR substantiate the different binding modes of imatinib/nilotinib and dasatinib. J. Biol. Chem. 283, 18292–18302 (2008).
Zhou, T. et al. Crystal structure of the T315I mutant of AbI kinase. Chem. Biol. Drug Des. 70, 171–181 (2007).
Modugno, M. et al. Crystal structure of the T315I Abl mutant in complex with the aurora kinases inhibitor PHA-739358. Cancer Res. 67, 7987–7990 (2007).
Carpinelli, P. et al. PHA-739358, a potent inhibitor of Aurora kinases with a selective target inhibition profile relevant to cancer. Mol. Cancer Ther. 6, 3158–3168 (2007).
Nagar, B. et al. Structural basis for the autoinhibition of c-Abl tyrosine kinase. Cell 112, 859–871 (2003).
Nagar, B. et al. Crystal structures of the kinase domain of c-Abl in complex with the small molecule inhibitors PD173955 and imatinib (STI-571). Cancer Res. 62, 4236–4243 (2002).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
Nathaneal Gray is a consultant for the Novartis Institute of Biomedical Research.
Supplementary information
Supplementary information S1 (table)
Kinases implicated in human cancer (PDF 265 kb)
Supplementary information S2 (figure)
The "DFG–in" conformation of EGFR (PDB ID 2J5F) with the covalent inhibitor 34–JAB (blue sticks) (PDF 143 kb)
Supplementary information S3 (box)
Kinases in the different groups (PDF 103 kb)
Supplementary information S4 (figure)
Developing inhibitors of mutant Bcr–Abl kinase (PDF 210 kb)
Related links
Related links
DATABASES
National Cancer Institute Drug Dictionary
Protein Data Bank
OMIM
FURTHER INFORMATION
Catalogue of somatic mutations in cancer
Glossary
- Synthetic lethal
-
Two genes have a synthetic-lethal phenotype if the individual gene deletions are not lethal but the combined mutation is. A synthetic-lethal interaction is proof of a genetic interaction.
- Heterocycle
-
An organic compound with a ring structure containing non-carbon atoms such as sulphur, oxygen or nitrogen as part of the ring.
- Pharmacophore
-
A molecular framework that carries the essential features responsible for the biological activity of a drug.
- Allosteric site
-
A site distinct from the enzyme active site that can bind a ligand that either positively or negatively regulates enzyme activity.
- Nucleophilic
-
A group of atoms that is rich in electrons and that participates in a chemical reaction by donating electrons to an electron-poor electrophile (electron lover).
- Electrophile
-
A group of atoms that is deficient in electrons and that participates in a chemical reaction by accepting electrons from an electron-rich nucleophile (nucleus lover).
- Michael addition
-
Nucleophilic addition to an α,β-unsaturated carbonyl compound. It belongs to the larger class of conjugate additions.
- Polyketide
-
Secondary metabolites from bacteria, fungi, plants and animals with diverse biological activities and pharmacological properties.
- Isostere
-
A chemical group with similar size and electronic properties.
- Homology model
-
A class of methods for constructing a three-dimensional structure of a target protein for which only the sequence is available, provided at least one empirical three-dimensional template structure with > 30% sequence identity is available.
- IC50
-
Inhibitor concentration for half-maximum response.
- EC50
-
Effector concentration for half-maximum response.
- Enrichment factor
-
The ratio of the number of biologically active compounds discovered using computationally based selection to the number found by random screening.
- Chemical space
-
The space spanned by all possible (that is, energetically stable) stoichiometrical combinations of electrons and atomic nuclei and topologies (isomers) in molecules and also compounds in general.
- Bisindolylmaleimide
-
Two fused indole rings bridged by a maleimide that can act as an ATP-competitive kinase inhibitor.
- Steric clash
-
Atoms contain an electron shell with a defined radius that prevents atoms not engaged in a covalent bond from occupying the same volume. A steric clash occurs when two atoms are placed closer than the sum of their atomic radii.
- Farnesyltransferase activity
-
Post-translational modification of proteins that consists of attaching an isoprenyl group to a cysteine residue.
Rights and permissions
About this article
Cite this article
Zhang, J., Yang, P. & Gray, N. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer 9, 28–39 (2009). https://doi.org/10.1038/nrc2559
Issue Date:
DOI: https://doi.org/10.1038/nrc2559
This article is cited by
-
Combined inhibition of HER2 and VEGFR synergistically improves therapeutic efficacy via PI3K-AKT pathway in advanced ovarian cancer
Journal of Experimental & Clinical Cancer Research (2024)
-
Novel meriolin derivatives activate the mitochondrial apoptosis pathway in the presence of antiapoptotic Bcl-2
Cell Death Discovery (2024)
-
Imaging the TGFβ type I receptor in pulmonary arterial hypertension
EJNMMI Research (2023)
-
Novel biomolecules in targeted cancer therapy: a new approach towards precision medicine
Medical Oncology (2023)
-
New Nano-Silica Supported Basic Ionic Liquids-Catalyzed Multicomponent Synthesis of imidazo [1,2-a] Pyridines Under Solvent-Free Conditions
Catalysis Letters (2023)