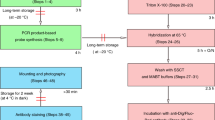
Abstract
Spatial information is critical to the interrogation of developmental and tissue-level regulation of gene expression. However, this information is usually lost when global mRNA levels from tissues are measured using reverse transcriptase PCR, microarray analysis or high-throughput sequencing. By contrast, single-molecule fluorescence in situ hybridization (smFISH) preserves the spatial information of the cellular mRNA content with subcellular resolution within tissues. Here we describe an smFISH protocol that allows for the quantification of single mRNAs in Drosophila embryos, using commercially available smFISH probes (e.g., short fluorescently labeled DNA oligonucleotides) in combination with wide-field epifluorescence, confocal or instant structured illumination microscopy (iSIM, a super-resolution imaging approach) and a spot-detection algorithm. Fixed Drosophila embryos are hybridized in solution with a mixture of smFISH probes, mounted onto coverslips and imaged in 3D. Individual fluorescently labeled mRNAs are then localized within tissues and counted using spot-detection software to generate quantitative, spatially resolved gene expression data sets. With minimum guidance, a graduate student can successfully implement this protocol. The smFISH procedure described here can be completed in 4–5 d.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Femino, A.M., Fay, F.S., Fogarty, K. & Singer, R.H. Visualization of single RNA transcripts in situ. Science 280, 585–590 (1998).
Raj, A., van den Bogaard, P., Rifkin, S.A., van Oudenaarden, A. & Tyagi, S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat. Methods 5, 877–879 (2008).
Eswaramoorthy, P. et al. Asymmetric division and differential gene expression during a bacterial developmental program requires DivIVA. PLoS Genet. 10, e1004526 (2014).
Little, S.C., Tikhonov, M. & Gregor, T. Precise developmental gene expression arises from globally stochastic transcriptional activity. Cell 154, 789–800 (2013).
Trcek, T. et al. Drosophila germ granules are structured and contain homotypic mRNA clusters. Nat. Commun. 6, 7962 (2015).
Little, S.C., Sinsimer, K.S., Lee, J.J., Wieschaus, E.F. & Gavis, E.R. Independent and coordinate trafficking of single Drosophila germ plasm mRNAs. Nat. Cell Biol. 17, 558–568 (2015).
Xu, H., Sepulveda, L.A., Figard, L., Sokac, A.M. & Golding, I. Combining protein and mRNA quantification to decipher transcriptional regulation. Nat. Methods 12, 739–742 (2015).
Trovisco, V. et al. icoid mRNA localises to the Drosophila oocyte anterior by random Dynein-mediated transport and anchoring. Elife http://dx.doi.org/10.7554/eLife.17537 (2016).
Lionnet, T. et al. A transgenic mouse for in vivo detection of endogenous labeled mRNA. Nat. Methods 8, 165–170 (2011).
York, A.G. et al. Instant super-resolution imaging in live cells and embryos via analog image processing. Nat. Methods 10, 1122–1126 (2013).
Curd, A. et al. Construction of an instant structured illumination microscope. Methods 88, 37–47 (2015).
Halstead, J.M. et al. Translation. An RNA biosensor for imaging the first round of translation from single cells to living animals. Science 347, 1367–1671 (2015).
Lecuyer, E., Necakov, A.S., Caceres, L. & Krause, H.M. High-resolution fluorescent in situ hybridization of Drosophila embryos and tissues. CSH Protoc. 2008 http://dx.doi.org/10.1101/pdb.prot5019 (2008).
Lecuyer, E., Parthasarathy, N. & Krause, H.M. Fluorescent in situ hybridization protocols in Drosophila embryos and tissues. Methods Mol. Biol. 420, 289–302 (2008).
Wilkie, G.S. & Davis, I. Visualizing mRNA by in situ Hybridization Using 'High Resolution' and Sensitive Tyramide Signal Amplification 94–97 (Academic Press, 1994).
Lecuyer, E. et al. Global analysis of mRNA localization reveals a prominent role in organizing cellular architecture and function. Cell 131, 174–187 (2007).
Buxbaum, A.R., Wu, B. & Singer, R.H. Single β-actin mRNA detection in neurons reveals a mechanism for regulating its translatability. Science 343, 419–422 (2014).
Trcek, T. et al. Single-mRNA counting using fluorescent in situ hybridization in budding yeast. Nat. Protoc. 7, 408–419 (2012).
Zenklusen, D. & Singer, R.H. Analyzing mRNA expression using single mRNA resolution fluorescent in situ hybridization. Methods Enzymol. 470, 641–659 (2010).
Sinsimer, K.S., Lee, J.J., Thiberge, S.Y. & Gavis, E.R. Germ plasm anchoring is a dynamic state that requires persistent trafficking. Cell Rep. 5, 1169–1177 (2013).
Davidson, A., Parton, R.M., Rabouille, C., Weil, T.T. & Davis, I. Localized translation of gurken/TGF-α mRNA during axis specification is controlled by access to Orb/CPEB on processing bodies. Cell Rep. 14, 2451–2462 (2016).
Gandhi, S.J., Zenklusen, D., Lionnet, T. & Singer, R.H. Transcription of functionally related constitutive genes is not coordinated. Nat. Struct. Mol. Biol. 18, 27–34 (2011).
Zenklusen, D., Larson, D.R. & Singer, R.H. Single-RNA counting reveals alternative modes of gene expression in yeast. Nat. Struct. Mol. Biol. 15, 1263–1271 (2008).
Nair, G., Walton, T., Murray, J.I. & Raj, A. Gene transcription is coordinated with, but not dependent on, cell divisions during C. elegans embryonic fate specification. Development 140, 3385–3394 (2013).
Castelnuovo, M. et al. Bimodal expression of PHO84 is modulated by early termination of antisense transcription. Nat. Struct. Mol. Biol. 20, 851–858 (2013).
Larkin, J.D. & Cook, P.R. Super-resolution measurement of distance between transcription sites using RNA FISH with intronic probes. Methods 98, 150–157 (2016).
Skinner, S.O., Sepulveda, L.A., Xu, H. & Golding, I. Measuring mRNA copy number in individual Escherichia coli cells using single-molecule fluorescent in situ hybridization. Nat. Protoc. 8, 1100–1113 (2013).
Itzkovitz, S. et al. Single-molecule transcript counting of stem-cell markers in the mouse intestine. Nat. Cell Biol. 14, 106–114 (2012).
Chen, K.H., Boettiger, A.N., Moffitt, J.R., Wang, S. & Zhuang, X. RNA imaging. Spatially resolved, highly multiplexed RNA profiling in single cells. Science 348, aaa6090 (2015).
Taniguchi, Y. et al. Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science 329, 533–538 (2010).
Battich, N., Stoeger, T. & Pelkmans, L. Image-based transcriptomics in thousands of single human cells at single-molecule resolution. Nat. Methods 10, 1127–1133 (2013).
Vargas, D.Y. et al. Single-molecule imaging of transcriptionally coupled and uncoupled splicing. Cell 147, 1054–1065 (2011).
Dong, S. et al. YRA1 autoregulation requires nuclear export and cytoplasmic Edc3p-mediated degradation of its pre-mRNA. Mol. Cell 25, 559–573 (2007).
Hoyle, N.P. & Ish-Horowicz, D. Transcript processing and export kinetics are rate-limiting steps in expressing vertebrate segmentation clock genes. Proc. Natl. Acad. Sci. USA 110, E4316–E4324 (2013).
Trcek, T., Sato, H., Singer, R.H. & Maquat, L.E. Temporal and spatial characterization of nonsense-mediated mRNA decay. Genes Dev. 27, 541–551 (2013).
Batish, M., van den Bogaard, P., Kramer, F.R. & Tyagi, S. Neuronal mRNAs travel singly into dendrites. Proc. Natl. Acad. Sci. USA 109, 4645–4650 (2012).
Weil, T.T. et al. Drosophila patterning is established by differential association of mRNAs with P bodies. Nat. Cell Biol. 14, 1305–1313 (2012).
Colak, D., Ji, S.J., Porse, B.T. & Jaffrey, S.R. Regulation of axon guidance by compartmentalized nonsense-mediated mRNA decay. Cell 153, 1252–1265 (2013).
Park, H.Y., Trcek, T., Wells, A.L., Chao, J.A. & Singer, R.H. An unbiased analysis method to quantify mRNA localization reveals its correlation with cell motility. Cell Rep. 1, 179–184 (2012).
Campbell, P.D., Chao, J.A., Singer, R.H. & Marlow, F.L. Dynamic visualization of transcription and RNA subcellular localization in zebrafish. Development 142, 1368–1374 (2015).
Besse, F., Lopez de Quinto, S., Marchand, V., Trucco, A. & Ephrussi, A. Drosophila PTB promotes formation of high-order RNP particles and represses oskar translation. Genes Dev. 23, 195–207 (2009).
Bahar Halpern, K. & Itzkovitz, S. Single molecule approaches for quantifying transcription and degradation rates in intact mammalian tissues. Methods 98, 134–142 (2016).
Messier, V., Zenklusen, D. & Michnick, S.W. A nutrient-responsive pathway that determines M phase timing through control of B-cyclin mRNA stability. Cell 153, 1080–1093 (2013).
Trcek, T., Larson, D.R., Moldon, A., Query, C.C. & Singer, R.H. Single-molecule mRNA decay measurements reveal promoter- regulated mRNA stability in yeast. Cell 147, 1484–1497 (2011).
Cabili, M.N. et al. Localization and abundance analysis of human lncRNAs at single-cell and single-molecule resolution. Genome Biol. 16, 20 (2015).
van Werven, F.J. et al. Transcription of two long noncoding RNAs mediates mating-type control of gametogenesis in budding yeast. Cell 150, 1170–1181 (2012).
Deng, W., Shi, X., Tjian, R., Lionnet, T. & Singer, R.H. CASFISH: CRISPR/Cas9-mediated in situ labeling of genomic loci in fixed cells. Proc. Natl. Acad. Sci. USA 112, 11870–11875 (2015).
Lubeck, E. & Cai, L. Single-cell systems biology by super-resolution imaging and combinatorial labeling. Nat. Methods 9, 743–748 (2012).
Levsky, J.M., Shenoy, S.M., Pezo, R.C. & Singer, R.H. Single-cell gene expression profiling. Science 297, 836–840 (2002).
Lubeck, E., Coskun, A.F., Zhiyentayev, T., Ahmad, M. & Cai, L. Single-cell in situ RNA profiling by sequential hybridization. Nat. Methods 11, 360–361 (2014).
Levesque, M.J. & Raj, A. Single-chromosome transcriptional profiling reveals chromosomal gene expression regulation. Nat. Methods 10, 246–248 (2013).
Lucy, L.B. Iterative technique for rectification of observed distributions. Astron. J. 79, 745–754 (1974).
Richardson, W.H. Bayesian-based iterative method of image restoration. J. Opt. Soc. Am. 62, 55–59 (1972).
Tyagi, S. & Kramer, F.R. Molecular beacons: probes that fluoresce upon hybridization. Nat. Biotechnol. 14, 303–308 (1996).
Bratu, D.P. Molecular beacons light the way: imaging native mRNAs in living cells. Discov. Med. 3, 44–47 (2003).
Mhlanga, M.M. et al. In vivo colocalisation of oskar mRNA and trans-acting proteins revealed by quantitative imaging of the Drosophila oocyte. PLoS One 4, e6241 (2009).
Chao, J.A., Patskovsky, Y., Almo, S.C. & Singer, R.H. Structural basis for the coevolution of a viral RNA-protein complex. Nat. Struct. Mol. Biol. 15, 103–105 (2008).
Hocine, S., Raymond, P., Zenklusen, D., Chao, J.A. & Singer, R.H. Single-molecule analysis of gene expression using two-color RNA labeling in live yeast. Nat. Methods 10, 119–121 (2013).
Abbaszadeh, E.K. & Gavis, E.R. Fixed and live visualization of RNAs in Drosophila oocytes and embryos. Methods 98, 34–41 (2016).
Park, H.Y., Buxbaum, A.R. & Singer, R.H. Single mRNA tracking in live cells. Methods Enzymol. 472, 387–406 (2010).
Buxbaum, A.R., Haimovich, G. & Singer, R.H. In the right place at the right time: visualizing and understanding mRNA localization. Nat. Rev. Mol. Cell Biol. 16, 95–109 (2015).
Forrest, K.M. & Gavis, E.R. Live imaging of endogenous RNA reveals a diffusion and entrapment mechanism for nanos mRNA localization in Drosophila. Curr. Biol. 13, 1159–1168 (2003).
Tyagi, S. Imaging intracellular RNA distribution and dynamics in living cells. Nat. Methods 6, 331–338 (2009).
Bertrand, E. et al. Localization of ASH1 mRNA particles in living yeast. Mol. Cell 2, 437–445 (1998).
Daigle, N. & Ellenberg, J. ambda(N)-GFP: an RNA reporter system for live-cell imaging. Nat. Methods 4, 633–636 (2007).
Garcia, J.F. & Parker, R. MS2 coat proteins bound to yeast mRNAs block 5′ to 3′ degradation and trap mRNA decay products: implications for the localization of mRNAs by MS2-MCP system. RNA 21, 1393–1395 (2015).
Gustafsson, M.G. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 198, 82–87 (2000).
Frise, E., Hammonds, A.S. & Celniker, S.E. Systematic image-driven analysis of the spatial Drosophila embryonic expression landscape. Mol. Syst. Biol. 6, 345 (2010).
Gavis, E.R. & Lehmann, R. Localization of nanos RNA controls embryonic polarity. Cell 71, 301–313 (1992).
Lehmann, R. Germ plasm biogenesis--an oskar-centric perspective. Curr. Top. Dev. Biol. 116, 679–707 (2016).
Jambor, H., Brunel, C. & Ephrussi, A. Dimerization of oskar 3′ UTRs promotes hitchhiking for RNA localization in the Drosophila oocyte. RNA 17, 2049–2057 (2011).
modEncode Consortium. Identification of functional elements and regulatory circuits by Drosophila modENCODE. Science 330, 1787–1797 (2010).
Ashburner, M. Drosophila (Cold Spring Harbor Laboratory, 1989).
Markow, T.A., Beall, S. & Matzkin, L.M. Egg size, embryonic development time and ovoviviparity in Drosophila species. J. Evol. Biol. 22, 430–434 (2009).
Gao, M. & Arkov, A.L. Next generation organelles: structure and role of germ granules in the germline. Mol. Reprod. Dev. 80, 610–623 (2013).
Mahowald, A.P. Fine structure of pole cells and polar granules in Drosophila melanogaster. J. Exp. Zool. 151, 201–215 (1962).
Voronina, E., Seydoux, G., Sassone-Corsi, P. & Nagamori, I. RNA granules in germ cells. Cold Spring Harb. Perspect. Biol. 3 http://dx.doi.org/10.1101/cshperspect.a002774 (2011).
Mahowald, A.P. Ultrastructural observations on oogenesis in Drosophila. J. Morphol. 137, 29–48 (1972).
Cho, W.K. et al. RNA polymerase II cluster dynamics predict mRNA output in living cells. Elife 5 http://dx.doi.org/10.7554/eLife.13617 (2016).
Raj, A., Peskin, C.S., Tranchina, D., Vargas, D.Y. & Tyagi, S. Stochastic mRNA synthesis in mammalian cells. PLoS Biol. 4, e309 (2006).
Lagha, M. et al. Paused Pol II coordinates tissue morphogenesis in the Drosophila embryo. Cell 153, 976–987 (2013).
Levsky, J.M., Shenoy, S.M., Pezo, R.C. & Singer, R.H. Single-cell gene expression profiling. Science 297, 836–840 (2002).
Pezo, R.C. et al. Single-cell transcription site activation predicts chemotherapy response in human colorectal tumors. Cancer Res. 68, 4977–4982 (2008).
Bothma, J.P., Magliocco, J. & Levine, M. The snail repressor inhibits release, not elongation, of paused Pol II in the Drosophila embryo. Curr. Biol. 21, 1571–1577 (2011).
de Turris, V., Nicholson, P., Orozco, R.Z., Singer, R.H. & Muhlemann, O. Cotranscriptional effect of a premature termination codon revealed by live-cell imaging. RNA 17, 2094–2107 (2011).
Acknowledgements
We thank the NYU Langone Medical Center (NYULMC) Microscopy Laboratory for providing the API DeltaVision personal DV microscope, particularly Y. Deng and M. Cammer for help with the wide-field epifluorescence microscope. We thank V. Schoonderwoert (Scientific Volume Imaging) for his help with the Huygens deconvolution software. This work was supported by the Intramural Research Programs of the US National Institute of Biomedical Imaging and Bioengineering. T.T. is a Howard Hughes Medical Institute (HHMI) fellow of the Jane Coffin Childs Memorial Fund. R.L. is an HHMI investigator. Funding for T.L. was provided by the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
T.T. and R.L. implemented quantitative smFISH in Drosophila tissue. H.S. developed iSIM. T.L. developed Airlocalize. All the authors wrote and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
Supplementary Table 1. List of sequences of all smFISH probes used in this study. (☼) denotes the position of the fluorophore in the sequence. (PDF 67 kb)
Rights and permissions
About this article
Cite this article
Trcek, T., Lionnet, T., Shroff, H. et al. mRNA quantification using single-molecule FISH in Drosophila embryos. Nat Protoc 12, 1326–1348 (2017). https://doi.org/10.1038/nprot.2017.030
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.030
This article is cited by
-
Microfluidics for understanding model organisms
Nature Communications (2022)
-
Spatial transcriptomics using combinatorial fluorescence spectral and lifetime encoding, imaging and analysis
Nature Communications (2022)
-
Nucleus size and DNA accessibility are linked to the regulation of paraspeckle formation in cellular differentiation
BMC Biology (2020)
-
Demystifying biotrophs: FISHing for mRNAs to decipher plant and algal pathogen–host interaction at the single cell level
Scientific Reports (2020)
-
Direct and simultaneous observation of transcription and chromosome architecture in single cells with Hi-M
Nature Protocols (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.