Abstract
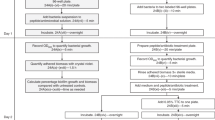
The incidence of fungal infections has increased significantly over the past decades. Very often these infections are associated with biofilm formation on implanted biomaterials and/or host surfaces. This has important clinical implications, as fungal biofilms display properties that are dramatically different from planktonic (free-living) populations, including increased resistance to antifungal agents. Here we describe a rapid and highly reproducible 96-well microtiter-based method for the formation of fungal biofilms, which is easily adaptable for antifungal susceptibility testing. This model is based on the ability of metabolically active sessile cells to reduce a tetrazolium salt (2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide) to water-soluble orange formazan compounds, the intensity of which can then be determined using a microtiter-plate reader. The entire procedure takes approximately 2 d to complete. This technique simplifies biofilm formation and quantification, making it more reliable and comparable among different laboratories, a necessary step toward the standardization of antifungal susceptibility testing of biofilms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Clark, T.A. & Hajjeh, R.A. Recent trends in the epidemiology of invasive mycoses. Curr. Opin. Infect Dis. 15, 569–574 (2002).
Dixon, D.M., McNeil, M.M., Cohen, M.L., Gellin, B.G. & La Montagne, J.R. Fungal infections: a growing threat. Public Health Rep. 111, 226–235 (1996).
Perlroth, J., Choi, B. & Spellberg, B. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med. Mycol. 45, 321–346 (2007).
Donlan, R.M. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8, 881–890 (2002).
Costerton, J.W., Stewart, P.S. & Greenberg, E.P. Bacterial biofilms: a common cause of persistent infections. Science 284, 1318–1322 (1999).
Ramage, G., Saville, S.P., Thomas, D.P. & Lopez-Ribot, J.L. Candida biofilms: an update. Eukaryot. Cell 4, 633–638 (2005).
Bachmann, S.P. et al. In vitro activity of caspofungin against Candida albicans biofilms. Antimicrob. Agents Chemother. 46, 3591–3596 (2002).
Kuhn, D.M., George, T., Chandra, J., Mukherjee, P.K. & Ghannoum, M.A. Antifungal Susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and Echinocandins. Antimicrob. Agents Chemother. 46, 1773–1780 (2002).
National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard. NCCLS Document M27-A. National Committee for Clinical Laboratory Standards (Wayne, PA, 1997).
Al-Fattani, M.A. & Douglas, L.J. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J. Med. Microbiol. 55, 999–1008 (2006).
Baillie, G.S. & Douglas, L.J. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob. Agents Chemother. 42, 1900–1905 (1998).
Baillie, G.S. & Douglas, L.J. Iron-limited biofilms of Candida albicans and their susceptibility to amphotericin B. Antimicrob. Agents Chemother. 42, 2146–2149 (1998).
Garcia-Sanchez, S. et al. Candida albicans biofilms: a developmental state associated with specific and stable gene expression patterns. Eukaryot. Cell. 3, 536–545 (2004).
Harrison, J.J., Turner, R.J. & Ceri, H. A subpopulation of Candida albicans and Candida tropicalis biofilm cells are highly tolerant to chelating agents. FEMS Microbiol. Lett. 272, 172–181 (2007).
Nobile, C.J. & Mitchell, A.P. C. albicans transcription factor Bcr1p: a regulator of cell-surface genes and biofilm formation. Current Biol. 15, 1150–1155 (2005).
Parahitiyawa, N.B. et al. Interspecies variation in Candida biofilm formation studied using the Calgary biofilm device. Apmis 114, 298–306 (2006).
Ramage, G., Bachmann, S., Patterson, T.F., Wickes, B.L. & Lopez-Ribot, J.L. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J. Antimicrob. Chemother. 49, 973–980 (2002).
Ramage, G., Tomsett, K., Wickes, B.L., Lopez-Ribot, J.L. & Redding, S.W. Denture stomatitis: a role for Candida biofilms. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 98, 53–59 (2004).
Ramage, G., Wickes, B.L. & Lopez-Ribot, J.L. A seed and feed model for the formation of Candida albicans biofilms under flow conditions using an improved modified Robbins device. Rev. Iberoam. Micol. 25, 37–40 (2008).
Suci, P.A., Geesey, G.G. & Tyler, B.J. Integration of Raman microscopy, differential interference contrast microscopy, and attenuated total reflection Fourier transform infrared spectroscopy to investigate chlorhexidine spatial and temporal distribution in Candida albicans biofilms. J. Microbiol. Methods 46, 193–208 (2001).
Vediyappan, G. & Chaffin, W.L. Non-glucan attached proteins of Candida albicans biofilm formed on various surfaces. Mycopathologia 161, 3–10 (2006).
Hawser, S.P., Norris, H., Jessup, C.J. & Ghannoum, M.A. Comparison of a 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-t etrazolium hydroxide (XTT) colorimetric method with the standardized National Committee for Clinical Laboratory Standards method of testing clinical yeast isolates for susceptibility to antifungal agents. J. Clin. Microbiol. 36, 1450–1452 (1998).
Honraet, K., Goetghebeur, E. & Nelis, H.J. Comparison of three assays for the quantification of Candida biomass in suspension and CDC reactor grown biofilms. J. Microbiol. Methods 63, 287–295 (2005).
Jin, Y. et al. The use of new probes and stains for improved assessment of cell viability and extracellular polymeric substances in Candida albicans biofilms. Mycopathologia 159, 353–360 (2005).
Ramage, G., Vande Walle, K., Wickes, B.L. & Lopez-Ribot, J.L. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob. Agents Chemother. 45, 2475–2479 (2001).
Tellier, R., Krajden, M., Grigoriew, G.A. & Campbell, I. Innovative endpoint determination system for antifungal susceptibility testing of yeasts. Antimicrob. Agents Chemother. 36, 1619–1625 (1992).
Kuhn, D.M., Balkis, M., Chandra, J., Mukherjee, P.K. & Ghannoum, M.A. Uses and limitations of the XTT assay in studies of Candida growth and metabolism. J. Clin. Microbiol. 41, 506–508 (2003).
Jin, Y., Samaranayake, L.P., Samaranayake, Y. & Yip, H.K. Biofilm formation of Candida albicans is variably affected by saliva and dietary sugars. Arch. Oral. Biol. 49, 789–798 (2004).
Martinez, L.R. & Casadevall, A. Susceptibility of Cryptococcus neoformans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 50, 1021–1033 (2006).
Mowat, E., Butcher, J., Lang, S., Williams, C. & Ramage, G. Development of a simple model for studying the effects of antifungal agents on multicellular communities of Aspergillus fumigatus. J. Med. Microbiol. 56, 1205–1212 (2007).
Bachmann, S.P. et al. Antifungal combinations against Candida albicans biofilms in vitro. Antimicrob. Agents Chemother. 47, 3657–3659 (2003).
Ramage, G., VandeWalle, K., Bachmann, S.P., Wickes, B.L. & Lopez-Ribot, J.L. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob. Agents Chemother. 46, 3634–3636 (2002).
Acknowledgements
Work in the laboratory is funded by Grant number 5R21DE017294 from the National Institute of Dental & Craniofacial Research (to J.L.L.-R.). The content is solely our responsibility and does not necessarily represent the official views of the NIDCR, the NIAID or the NIH. A.R.T. was supported by the McNair Scholars Program at St Mary's University funded by the US Department of Education.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pierce, C., Uppuluri, P., Tristan, A. et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc 3, 1494–1500 (2008). https://doi.org/10.1038/nprot.2008.141
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2008.141
This article is cited by
-
Antimicrobial polyketides from Magellan Seamount-derived fungus Talaromyces scorteus AS-242
The Journal of Antibiotics (2023)
-
Discovery of prenylated indole alkaloid and natural xanthone from cold-seep sediment derived fungus Talaromyces funiculosus SD-523
Journal of Oceanology and Limnology (2023)
-
In silico molecular modelling studies and antibiofilm efficacy of shikonin against Candida albicans: mechanistic insight
Archives of Microbiology (2023)
-
Constituents, Antibacterial Effect, and Cytotoxicity of Essential Oil from Aerial Parts of Notopterygium incisum
Current Microbiology (2023)
-
A New Phthalide Derivative from the Mangrove-Derived Fungus Eupenicillium sp. HJ002
Chemistry of Natural Compounds (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.