Abstract
Acute hypercapnia (elevated arterial CO2/H+) is a suffocation signal that is life threatening and rapidly mobilizes adaptive changes in breathing and behavioral arousal in order to restore acid-base homeostasis. Severe hypercapnia, seen in respiratory disorders (eg, asthma or bronchitis, chronic obstructive pulmonary disease (COPD)), also results in high anxiety and autonomic activation. Recent evidence has demonstrated that wake-promoting hypothalamic orexin (ORX: also known as hypocretin) neurons are highly sensitive to local changes in CO2/H+, and mice lacking prepro-ORX have blunted respiratory responses to hypercapnia. Furthermore, in a recent clinical study, ORX-A, which crosses blood–brain barrier easily, was dramatically increased in the plasma of patients with COPD and hypercapnic respiratory failure. This is consistent with a rodent model of COPD where chronic exposure to cigarette smoke led to a threefold increase in hypothalamic ORX-A expression. In the present study, we determined the role of ORX in the anxiety-like behavior and cardiorespiratory responses to acute exposure to a threshold panic challenge (ie, 20% CO2/normoxic gas). Exposing conscious rats to such hypercapnic, but not atmospheric air, resulted in respiratory, pressor, and bradycardic responses, as well as anxiety-like behavior and increased cellular c-Fos responses in ORX neurons. Systemically, pre-treating rats with a centrally active ORX1 receptor antagonist (30 mg/kg SB334867) attenuated hypercapnic gas-induced pressor and anxiety responses, without altering the robust bradycardia response, and only attenuated breathing responses at offset of the CO2 challenge. Our results show that the ORX system has an important role in anxiety and sympathetic mobilization during hypercapnia. Furthermore, ORX1 receptor antagonists may be a therapeutic option rapidly treating increased anxiety and sympathetic drive seen during panic attacks and in hypercapnic states such as COPD.
Similar content being viewed by others
INTRODUCTION
Blood CO2/H+ is maintained within a very narrow range, and mild arterial elevations of CO2 (ie, hypercapnia), which can occur from hypoventilation or in some respiratory disorders, initially leads to an increase in respiratory activity to help ‘blow off’ excess CO2 (for review, see Guyenet et al, 2010). However, as CO2 levels continue to increase, adaptive behavioral, autonomic, and neuroendocrine responses occur. For instance, exposing rats to mild hypercarbic gas (eg, 7% CO2; Akilesh et al, 1997) increases respiratory activity that reduces hypercapnia without mobilizing other components of a ‘panic-like’ response. However, exposing rats to higher concentrations of hypercarbic gas (eg, ⩾10% CO2) elicits additional components of a panic-associated responses as evidenced by increases in sympathetic activity (Elam et al, 1981), blood pressure (Walker, 1987), anxiety-like behaviors (Cuccheddu et al, 1995; Johnson et al, 2011), and mobilization of the hypothalamic–pituitary–adrenal axis (Marotta et al, 1976; Sithichoke et al, 1978; Sithichoke and Marotta, 1978). In humans, a single breath of air containing 35% CO2 increases anxiety and sympathetic-adrenal responses (Griez and Van den Hout, 1983; Argyropoulos et al, 2002; Kaye et al, 2004) and inhaling 7.5% CO2 for 20 min also leads to increases in anxiety and cardiorespiratory responses (Bailey et al, 2005). Therefore, understanding the neural mechanisms underlying severe hypercapnia-induced anxiety and autonomic hyperactivity that can occur in chronic obstructive pulmonary disease (COPD), asthma, or bronchitis could lead to novel treatments for these symptoms. Yet, the neural circuits and associated neurochemicals by which high CO2 levels elicit panic-like responses are poorly understood.
Carbon dioxide readily crosses the blood–brain barrier (Fukuda et al, 1989; Forster and Smith, 2010) to directly interact with specialized CO2/H+ chemosensory neurons in the medulla with a high chemosensitivity and that are critical for regulating breathing following subtle changes in CO2/H+ (Guyenet et al, 2010). Orexin (ORX) neuropeptide-producing neurons, which are found only in the dorsomedial/perifornical (DMH/PeF) and adjacent lateral hypothalamus (LH) (Peyron et al, 1998) also display CO2/H+-sensitive properties, but with lesser chemosensitivity (Williams et al, 2007), suggesting that they may respond to only panic threshold hypercapnia. The endogenous ligands of the ORX precursor are ORX-A (which is completely conserved among several mammalian species) and ORX-B. Compared with ORX-B, ORX-A binds with a higher affinity to the ORX1 receptor, which is the receptor targeted here (for review, see Sakurai, 2007). This ORX system is critical for maintaining wakefulness (for review, see Sakurai, 2007) and a hyperactive ORX system is linked to: a rodent model of COPD (Liu et al, 2010); clinical COPD with hypercapnic respiratory disorder (Zhu et al, 2011); and anxiety- and panic-vulnerability in rats and humans (Johnson et al, 2010). Furthermore, during wakefulness prepro-ORX knockout mice have blunted respiratory responses to 5–10% hypercarbic gas exposure, and injecting wild-type mice with an ORX1 receptor antagonist attenuates hypercapnic-induced respiratory responses (Deng et al, 2007). The present studies in rats attempted to determine the role that ORX has in the behavioral and cardiovascular responses to an acute 20% CO2/normoxic gas challenge (a stimulus where ambient CO2 concentrations rise and peak just at the 5-min point, then rapidly decreases at offset; Johnson et al, 2005).
MATERIALS AND METHODS
Animals and Housing Conditions
All experiments were conducted on adult male Sprague–Dawley rats (300–350 g) purchased from Harlan Laboratories (Indianapolis, IN; experiments 1, 3, and 4 and Barcelona, Spain for experiment 2) and were housed individually in plastic cages under standard environmental conditions (22 °C; 12–12 light–dark cycle; lights on at 0700 hours) for 7–10 days before the surgical manipulations. Food and water were provided ad libitum. Animal care procedures were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals (NIH Publication no. 80–23) revised 1996 and the guidelines of the IUPUI Institutional Animal Care and Use Committee for experiments 1, 3, and 4 and the Instituto Cajal, Consejo Superior de Investigaciones Científicas (CSIC), Madrid, Spain for experiment 2.
Experiment 1: Effects of Hypercarbic Gas on Behavior and Cardiovascular Activity in Conscious Rats
Surgical procedures for telemetry probe implantation
Before surgery, rats were anesthetized with a nose cone connected to an isoflurane system (MGX Research Machine; Vetamic, Rossville IN) during the surgery. All rats were fitted with femoral arterial catheters for measurement of mean arterial blood pressure (MAP) and heart rate (HR) as previously described (Shekhar et al, 1996). Briefly, cardiovascular responses were measured by a femoral arterial line connected to a telemetric probe that contained a pressure transducer (Cat. no. C50-PXT, Data Sciences International (DSI), St Paul, MN). DSI DATAQUEST software was used to monitor and record MAP and HR. For the duration of each experiment, MAP and HR were recorded continuously in freely moving conscious rats. Data were analyzed during the period 5 min before, 5 min during, and 5 min following the gas challenges. The data reported are changes in HR and MAP, expressed in 1-min bins, relative to the average of the baseline measurement (t−5 min to t−1 min) from each rat.
Description of hypercarbic or atmospheric gas infusion
Flow cages (30.5 cm width × 30.5 cm height × 61 cm length) were custom built using Plexiglas. When the lid of the cage was latched, gases could only enter the cage through an inlet connector (for the gas infusion) and could only exit the cage through an outlet connector. The gas flow into the cages was controlled using a two-stage regulator (Praxair, Danbury, CT) at a pressure of 0.6 bar. We previously validated the consistency of the rate of CO2 delivery using state-of-the-art infrared CO2 (ProCO2) and electrochemical O2 (ProO2) sensors (Johnson et al, 2005). Specifically, concentrations of O2 remain at 21% throughout the gas infusion in the control and experimental cages (see Johnson et al, 2005). The CO2 concentration remains constant at <1% in the control cage during exposure of rats to atmospheric air (<1% CO2/21% O2/79% N2). Infusion of the premixed normoxic, hypercarbic gas (20% CO2/21% O2/59% N2) results in a rapid increase in CO2 concentration from <1% CO2 up to 20% CO2 at the 5-min time point. After terminating gas infusion and opening the cages, the concentration of CO2 rapidly decreases from 20% to <2.5% CO2 during the following 5 min. Using a portable iSTAT gas analyzer (HESKA, Des Moines, IA), we have also determined that this hypercapnic, normoxic challenge leads to arterial PCO2/pH levels of ∼130 mmHg/7.01 during the challenge that are back to normal physiological range (∼50 mmHg/7.37) within 2-min post challenge (unpublished data).
On day 1, rats (n=3 per group) were selected from their home cages and placed into experimental cages containing atmospheric air. All rats had infusions of the following: (1) 5-min infusion of premixed atmospheric gas (<1% CO2, 21% O2, 79% N2: Praxair) for baseline measurements, then (2) either the premixed atmospheric control gas (<1% CO2, 21% O2, 79% N2) or premixed experimental normoxic, hypercarbic gas (20% CO2, 21% O2, 59% N2: Praxair) for 5 min (note: for control rats the atmospheric gas was turned off and back on again at the beginning and end of this infusion to be identical to the manipulations for the hypercarbic gas challenge), and finally, (3) 5-min infusion of atmospheric gas. Fecal pellets were counted in cages at the end of gas challenges. In order to assess anxiety-like behavioral responses following exposure to hypercarbic gas, rats were immediately transferred to an adjacent room and place in the center square of an open-field box for a 5-min test (see next section for details). On day 2, the experiment was repeated, but the treatments were reversed for each rat.
Open-field behavior test and analyses
The open-field arena covered an area of 90 cm × 90 cm, with 40 cm high walls. The open-field arena was divided into a 6 × 6 grid of equally sized squares using black tape (36 total squares) with 4 squares forming the center, 12 squares forming the middle perimeter, and 20 squares forming the outer perimeter. The test started by placing a rat in the center. The behavior of each rat in the open-field arena was recorded on video and scored afterward by an observer (PLJ) blind to the experimental treatment of each rat. Time spent in each region of the open-field was recorded. In addition, locomotor activity was assessed by counting the number of times the rat's entire body (excluding tail) completely crossed into another square.
Experiment 2: Effects of Hypercarbic Gas on Cellular Responses in ORX Neurons
Description of hypercarbic or atmospheric gas infusion
All rats received hypercarbic or atmospheric gas infusions as described in detail in experiment 1 then were transferred to their original home cages 5 min following offset of gas exposure (n=7 per group). To validate the consistency of the rate of CO2 delivery, we monitored CO2 and O2 concentrations within the experimental flow cages using state-of-the-art infrared CO2 (ProCO2) and electrochemical O2 (ProO2) sensors (Biospherix, Redfield, NY).
Perfusion
At 90 min following the initiation of treatment, rats were anesthetized with an overdose of sodium pentobarbital (40 mg, i.p.), then perfused transcardially with 0.05 M phosphate-buffered saline (PBS; 250 ml), followed by 0.1 M sodium phosphate buffer (PB; 250 ml) containing 4% paraformaldehyde and 3% sucrose and the brains were removed and processed for immunohistochemistry as described in detail previously (Johnson et al, 2011).
Immunohistochemistry
Double immunostaining for c-Fos protein and ORX was accomplished with sequential immunohistochemical procedures using (1) primary antibodies directed against c-Fos (rabbit anti-c-Fos polyclonal antibody, Cat. no. sc-52, Ab-5, Santa Cruz Biotechnology, Santa Cruz, CA; diluted 1 : 10 000) then (2) primary antibodies directed against ORX-A (rabbit anti-ORX-A-polyclonal, affinity-purified antibody, Cat. no. H-003-30, Phoenix Pharmaceuticals, Burlingame, CA; diluted 1 : 10 000). All brain sections were immunostained in a single immunohistochemical run, rather than in batches, with large volume incubations to limit variability in the quality of immunohistochemical staining among brain sections.
Free-floating sections were washed in 0.05 M PBS for 30 min, then incubated in 1% H2O2 in PBS for 20 min. Sections were then washed 10 min in PBS and 20 min in PBS with 0.3% Triton X-100 (PBST). Sections were then incubated 12–16 h in PBST with primary antibody solution at room temperature. After a 30-min wash in PBST, sections were incubated in biotinylated goat anti-rabbit IgG (c-Fos, ORX-A; Cat. no. BA-1000; Vector Laboratories, Burlingame, CA; diluted 1:500). Sections were washed again for 30 min in PBST then incubated 1.5 h in an avidin–biotin complex provided in a standard Vector Elite kit (c-Fos, ORX-A, Cat. no. PK-6100, Vector Laboratories; diluted 1:500). Substrates for chromogen reactions were SG (c-Fos; SK-4700, Vector Laboratories) or 0.01% 3,3′-diaminobenzidine tetrahydrochloride (ORX-A; DAB) (Cat. no. D-5637, Sigma-Aldrich, Poole, UK) in PBS containing 0.003% H2O2, pH 7.4. Substrate reactions were run for 20 min for c-Fos and 10 min for ORX-A. All sections were mounted on clean glass slides, dried overnight, dehydrated, and mounted with cover slips using DPX mounting medium (BDH Laboratory Supplies, Poole, UK). All washes and incubations were done in 12-well polystyrene plates with low-frequency shaking on an orbital shaker.
Counting of ORX-A- and c-FOS-ir neurons in experiment 2
Selection of anatomical levels for analysis of c-Fos/ORX-A-immunostained cells was conducted with reference to illustrations from a rat brain stereotaxic atlas (Paxinos and Watson, 1997). Selection of anatomical levels was also done in reference to major anatomical landmarks including white matter tracts and the ventricular systems. Specifically, darkfield contrast (ie, using a 1.6 × Leica phase contrast Plan objective and Leica binocular microscope (model DMLB, Leica Mikroskopie and Systeme GmbH, Wetzler, Germany) with a darkfield condenser) was used to visualize white matter tracts (eg, the fornix and optic tracts) and ventricular systems (eg, lateral, third ventricles) that aided in selection of appropriate coronal levels with reference to illustrations in a standard stereotaxic atlas of the rat brain (Paxinos and Watson, 1997). The numbers of c-Fos/ORX-A-ir neurons were counted in the entire field of view at × 400 magnification (ie, × 10 eyepiece and × 40 Plan objective) for each brain region. The area of the DMH/PeF where single ORX-A-ir neurons and double c-Fos/ORX-ir neurons was counted was roughly square in dimension with the corners being the mammillothalamic tract, the fornix, the top of the third ventricle and a point located halfway down the third ventricle (immediately medial from the fornix). The DMH/PeF, as described, is particularly sensitive to BMI-induced cardioexcitatory response (Samuels et al, 2004). All single ORX-A-ir neurons and double c-Fos/ORX-ir neurons counted that were lateral to the DMH/PeF area were considered to be in the LH region. All cell counts were done by an observer (PLJ) that was blind to the experimental treatment of each animal.
Photography
Photomicrographs were obtained using a brightfield microscope using N Plan 5 × , 10 × , 40 × , and 63 × objective lenses (Leica binocular microscope, model DMLB), an Insight digital camera (Diagnostics Instruments, Sterling Heights, Michigan) and SPOT 3.5.5 for Windows digital imaging software (Silicon Graphics, Mountain View, CA). Photographic plates were prepared in CorelDraw 11.633 for Windows.
Experiment 3: Effects of an ORX1 Antagonist on Hypercarbic Gas-Induced Changes in Behavior and Cardiovascular Activity in Conscious Rats
All rats received hypercarbic gas infusions as described in detail in experiment 1. However, 30 min before the hypercarbic gas challenge rats were injected with vehicle (0.2 ml/100 g volume dimethyl sulfoxide (DMSO)) or a dose of an ORX1 receptor antagonist (30 mg/kg SB334867, Tocris Bioscience, Bristol, UK, in 0.2 ml/100 g volume DMSO, i.p.) that blocks stress-induced anxiety-like behavior and panic-associated cardioexcitatory responses without inducing somnolence (Johnson et al, 2010). This drug crosses the blood–brain barrier (Ishii et al, 2005) and does not alter MAP, HR, or locomotor activity in control rats (Johnson et al, 2010). Blood pressure, HR, locomotor activity, number of fecal pellets, and anxiety-like behavior were assessed as described in experiment 1.
Experiments 4–5: Effects of an ORX1 Antagonist on Hypercarbic Gas-Induced Changes in Respiration Rate in Conscious Freely Moving Rats
Experiment 4—all rats received hypercarbic or atmospheric gas infusions as described in detail in experiment 1, with the following exceptions. Instead of being placed in a flow cage, rats were placed in a clear custom built Plexiglass cylindrical plethysmograph chamber (i.d. 95 mm, length 260 mm, volume 1.84 l, and wall thickness 3 mm) with atmospheric infused at a flow rate of 2.5 l/min until a steady baseline respiration rate was noted (approximately 30 min). A plastic T-connector was inserted 20 cm away from the start of the output line and then linked to one input of a differential pressure amplifier (model 24PC01SMT, Honeywell Sensing and Control, Golden Valley, MN), the second input being opened to the room air. The atmospheric infusion rate is sufficient to prevent any rise of CO2 in the plethysmograph (Kabir et al, 2010).
Experiment 5—30 min before the hypercapnic gas challenge, rats were either injected with vehicle (0.2 ml/100 g volume DMSO i.p., n=6) or an ORX1 receptor antagonist (30 mg/kg SB334867, Tocris Bioscience, in 0.2 ml/100 g volume DMSO i.p., n=6). All rats received a hypercarbic gas infusion as described in detail in experiment 1, and respiration rate was assessed using whole body plethysmography as described in experiment 4.
Experiments 6–7: Effects of an ORX1 Antagonist on Hypercarbic Gas-Induced Changes in Respiration Rate in Anesthetized Rats
Experiment 6—in light of motor–respiratory interactions (Kabir et al, 2010) in experiments 4–5, in experiments 6–7 we determined the effects of 20% CO2/normoxic gas on respiration rate in anesthetized rats and whether and ORX 1 receptor antagonist could alter this respiratory response. Rats were anesthetized with an i.p. injection of ketamine (5 mg/kg) then were placed in a plethysmographic chamber where baseline respiration rate was assessed for 30 min during atmospheric air challenge, then for 30 min with a hypercapnic (20% CO2), normoxic gas challenge.
Experiment 7—rats were anesthetized with an i.p. injection of ketamine (5 mg/kg) then were either injected with vehicle (0.2 ml/100 g volume DMSO i.p., n=6) or an ORX1 receptor antagonist (30 mg/kg SB334867, Tocris Bioscience, in 0.2 ml/100 g volume DMSO i.p., n=6) 30 min before a 20% CO2/normoxic gas challenge. All rats received a hypercarbic gas infusion as described in detail in experiment 6, and respiration rate was assessed using whole body plethysmography.
Statistical Analyses
Analyses of cardiovascular and respiratory responses and open-field behavior
Dependent variables for analyses of cardiovascular (HR, MAP), respiratory (rate and depth), and locomotor activity were analyzed using a one-way ANOVA with repeated measures, using gas infusion as the between-subjects factor and time as a within-subjects factor. Dependent variables for the number of fecal pellets and open-field test (ie, time spent in each section, line crossings) were analyzed using a one-way ANOVA with gas infusion in experiment 1 and drug treatment in experiment 3 as the between-subjects factors. In the presence of significant main effects or main effect × time interactions, Fisher's least significant difference (LSD) or paired t-tests were used for post-hoc pairwise comparisons because each rat received both atmospheric and hypercarbic gas infusions (experiments 1, 4, and 6) or vehicle+hypercarbic gas or SB334867+hypercarbic gas (experiments 3, 5, and 7) on different days. Within-subjects comparisons were also made on the cardiovascular and respiratory measures using a Dunnett's test for multiple comparisons with a single control using the 5-min baseline measurement as the control. The alpha level was set at 0.05 in all cases.
Statistical analyses of single ORX-ir and double c-FOS/ORX-ir neurons
The dependent variables for cell counts (number of single ORX-A-ir and double c-Fos/ORX-A-ir cells) were analyzed using a one-way ANOVA with gas exposure as the between-subjects factor and hypothalamic region as the repeated measure. In the presence of significant main effects or main effect × brain region interactions, post-hoc tests were conducted to define the anatomical location of the effects using an unpaired two-tailed t-test.
All statistical analyses were carried out using SYSTAT 5.02. (SYSTAT, San Jose, CA) and SPSS 14.0 (SPSS, Chicago, IL), and all graphs were generated using SigmaPlot 2001 (SPSS Inc) and an illustration program (CorelDraw 11.633 for Windows, Viglen, Alperton, UK).
RESULTS
Experiment 1: Cardiovascular and Behavioral Responses to Gas Infusions
Infusion of hypercarbic, but not atmospheric, gas increased MAP (gas infusion × time interaction, F(14,56)=6.4, P=0.0001; gas infusion effect, F(1,4)=11.0, P=0.029; CO2 group within-group time effect, F(14,30)=3.3, P=0.003, Figure 1a) and decreased HR (gas infusion × time interaction F(14,56)=2.4, P=0.011; CO2 group within-group time effect F(14,30)=3.1, P=0.005, Figure 1b) without altering locomotor activity (gas infusion × time interaction F(1,4)=2.5, P=0.200; data not shown). A 5-min atmospheric gas challenge did not alter MAP (Figure 1a), HR (Figure 1b) or locomotor (not shown) responses relative to the 5-min baseline. Rats exposed to hypercarbic gas also had increased numbers of fecal pellets post challenge, compared with control rats challenged with atmospheric gas (t(2)=−4.2, P=0.027, Figure 1c). No significant differences in baseline MAP (t(2)=3.1, P=0.090), HR (t(2)=0.8, P=0.764), or activity (t(2)=0.9, P=0.457) were noted between treatment groups during the initial 5-min baseline before challenge with experimental gases.
Graphs illustrate changes in (a) MAP and (b) HR during the gas infusion challenge (0 to +5 min, see grey shading) as compared with the 5-min baseline (−5 to 0 min). (c) Graph illustrating the effects of hypercapnic gas infusions on number of fecal pellets deposited during the 5-min test. (d) Graphs illustrate the open-field test results (ie, from left to right the graphs indicate the time spent in the outer and middle perimeter and center of the open field). #P<0.05; within-subjects effects of hypercapnia over time using a Dunnett's one-way test using t−1 min as baseline. *P<0.05, paired t-tests. Atm, atmospheric.
Open-field test
Compared with atmospheric gas-challenged rats, hypercarbic gas-challenged rats spent less time in the middle perimeter (t(2)=5.4, P=0.016) and more time in the outer perimeter (t(2)=−3.5, P=0.036) of the open-field (Figure 1d). No difference was noted between groups for the time spent in the center (t(2)=1.0, P=0.211) of the open field.
Experiment 2: Effects of Brief Hypercarbic Gas Exposure on c-Fos Induction in ORX Neurons
Rats exposed to hypercarbic gas had greater numbers of c-Fos/ORX-A-ir neurons in the DMH/PeF, but not LH, as compared with rats exposed to atmospheric air DMH/PeF (−2.94 mm. bregma: gas infusion × region interaction, F(1,12)=10.5, P=0.007; −3.12 mm bregma: gas infusion × region interaction, F(1,12)=11.1, P=0.006). The increase in c-Fos/ORX-A-ir neurons occurred in the DMH/PeF (−2.94 mm bregma: F(1,12)=11.2, P=0.006 Figure 2a and b; −3.12 mm bregma: F(1,12)=12.5, P=0.004, Figure 2c), but no effect was observed in the LH (−2.94 mm bregma: F(1,12)=1.8, P=0.206 Figure 2b; −3.12 mm bregma: F(1,12)=2.4, P=0.145, Figure 2c). There was no significant effect of gas exposure (−2.94 mm bregma: gas infusion × region interaction, F(1,12)=2.9, P=0.114; −3.12 mm bregma: gas infusion × region interaction, F(1,12)=0.02, P=0.901) on total numbers of ORX-A-ir neurons in either the DMH/PeF (−2.94 mm bregma: F(1,12)=0.8, P=0.389 Figure 2b; −3.12 mm bregma: F(1,12)=0.3, P=0.564, Figure 2c) or LH (−2.94 mm bregma: F(1,12)=1.4, P=0.266 Figure 2b; −3.12 mm bregma: F(1,12)=1.1, P=0.304, Figure 2c).
Effects of brief hypercarbic gas exposure on c-Fos expression in ORX-A-ir neurons. (a) Photographs of ORX-A cytoplasmic brown immunostained neurons with and without nuclear black immunostained nuclei. Arrows indicate double labelling. Scale bar indicates 25 μm. (b, c) Graphs illustrate the number of ORX neurons that expressed c-Fos (black lined bars), and the total number of ORX neurons present (gray lined bars). *P<0.05, unpaired t-test. 3V, third ventricle; DMH, dorsomedial hypothalamus; f, fornix; LH, lateral hypothalamus; mt, mammillothalamic tract; PeF, perifornical hypothalamus.
Experiment 3: Effect of an ORX1 Receptor Antagonist on Cardiovascular and Behavioral Responses to Hypercarbic Gas Infusions
Prior i.p. injections of SB334867, but not vehicle, attenuated hypercarbic gas-induced changes in MAP (drug × time interaction F(14,182)=6.4, P=0.0001; drug treatment effect F(1,13)=11.0, P=0.029; the veh/CO2, but not SB/CO2, group had a within-group time effect F(14,105)=2.6, P=0.003, Figure 3a), but unexpectedly had no effect on hypercarbic gas-induced bradycardia (drug × time interaction F(14,182)=0.5, P=0.931; drug treatment effect F(1,13)=1.0, P=0.346, with both the veh/CO2 (F(14,105)=9.8, P<0.001), and SB/CO2 (F(14,90)=8.9, P<0.001) group having a within-group time effect, Figure 3a and b). Neither the vehicle nor SB334867-treated rats had a change in locomotor responses over time before, during, or after hypercarbic gas (data not shown). Vehicle-treated rats exposed to hypercarbic gas had increased numbers of fecal pellets, relative to atmospheric gas-challenged control rats, which was attenuated by SB334867 (SB/CO2 group: F(2,21)=5.6, P=0.012, Figure 3c). No significant differences in baseline MAP (t(6)=−1.2, P=0.257) or HR (t(6)=0.3, P=0.770) were noted between treatment groups over the initial 5-min baseline before experimental gas challenges. However, rats pre-treated with SB334867 did have higher locomotor activity before hypercarbic gas infusion than the vehicle-treated rats (t(6)=−2.6, P=0.039). The hypercarbic gas-treated group only had an n of 7 because of a malfunctioning telemetry probe sending MAP and HR readings outside of the physiological range on the last test day.
Graphs illustrate changes in (a) MAP and (b) HR during the atmospheric or hypercapnic/normoxic gas infusion challenges (0 to +5 min, see grey shading) as compared with the 5-min baseline (−5 to 0 min). #Within-subjects effects of hypercapnic challenge over time using a Dunnett's one-way test using t−1 min as baseline; *P<0.05, between-subjects paired t-tests in Figure 3a. (c) Graph indicates number of fecal pellets from each group; *P<0.05, Fisher's LSD test, (d) graphs illustrating the open-field test results (ie, from left to right the graphs indicate the time spent in the outer and middle perimeter and center of the open-field). *P<0.05, Fisher's LSD test; #P<0.05, paired t-tests in, Figure 3d. Atm, atmospheric; SB, SB334867; Veh, vehicle.
Open-field test
Vehicle-treated rats exposed to hypercarbic gas spent less time in the middle perimeter area than vehicle-treated rats exposed to atmospheric air (F(2,21)=3.6, P=0.045, Figure 3d). When comparing all three groups, differences between the Veh/CO2 and the SB/CO2 group approached significance with a Fisher's LSD post-hoc test (P=0.056) that was protected by the previous ANOVA result. However, comparing the Veh/CO2 group with the SB/CO2 group with an unpaired t-test revealed that the SB/CO2 group spent significantly more time in the middle perimeter region than the Veh/CO2 group (t(7)=−2.7, P=0.016). No differences in the time spent in the center (F(2,21)=0.2, P=0.790) or outer perimeter (F(2,21)=0.2, P=0.085) regions were noted.
Experiments 4, 6: Effects of Hypercarbic Gas on Respiration Rate in Conscious or Anesthetized Rats
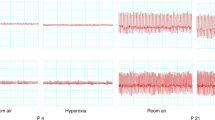
In conscious rats that can voluntarily control respiratory motor activity, the infusion of hypercarbic, but not atmospheric, gas increased respiration rate (gas infusion × time interaction, F(14,140)=3.4, P<0.001; and CO2 group within-group time effect F(5,89)=5.6, P<0.001, Figure 4a). In anesthetized rats, infusion of hypercarbic, but not atmospheric, gas decreased respiration rate (gas infusion × time interaction, F(59,236)=5.3, P<0.001; and CO2 group within-group time effect F(59,180)=6.9, P<0.001, Figure 4b). The hypercapnia-induced reduction in respiration rate seen in rats anesthetized with ketamine is opposite to that seen in conscious rats. This dramatically different effect is most likely a combined effect of ketamine on central arousal system (anesthetic effect), opioid systems (analgesic effect), but also on anxiety circuits, because low doses of ketamine have anxiolytic properties in humans (Irwin and Iglewicz, 2010).
Line graphs illustrate respiration rate (b.p.m., using whole body plethysmography) in (a) conscious (b) and anesthetized rats during an atmospheric or hypercapnic/normoxic gas challenge as compared with the baseline atmospheric air challenge. Line graphs illustrate respiration rate (b.p.m., using whole body plethysmography) in (c) conscious (d) and anesthetized rats during a hypercapnic/normoxic gas challenge as compared with the baseline atmospheric air challenge following an intraperitoneal injection with either a vehicle or 30 mg/kg dose of SB334867 30 min before the hypercapnic/normoxic gas infusion. *Within-subjects effects of hypercapnic challenge over time using a Dunnett's two-way test using t−1 min as baseline; #P<0.05, between-subjects paired t-tests in. Atm, atmospheric; SB, SB334867; Veh, vehicle.
Experiments 5, 7: Effects of an ORX1 Antagonist on Hypercarbic Gas-Induced Changes in Respiration Rate in Conscious or Anesthetized Rats
As observed in experiment 4, the hypercarbic gas challenge in conscious rats increased respiration rate over time in the vehicle pre-treated group (F(5,89)=17.4, P<0.001) and in the ORX1 receptor antagonist group (F(5,89)=4.6, P<0.001). A drug × time interaction F(14,140)=4.2, P<0.001 did occur, but post-hoc analyses revealed that the ORX1 receptor antagonist only altered respiratory responses following the offset of the hypercapnia gas challenge (Figure 4c).
As observed in experiment 6, the hypercarbic gas challenge in anesthetized rats decreased respiration rate over time in the vehicle pre-treated group (F(59,295)=17.0, P<0.001), but also in the ORX1 receptor antagonist group (F(59,295)=17.8, P<0.001). There was no drug × time interaction (F(59,590)=0.7, P=0.972, Figure 4d).
DISCUSSION
The studies presented here report, for the first time, the role of ORX in anxiety-associated behavior and cardio-respiratory responses to a brief (5 min) exposure to a high (20% CO2) concentration of hypercarbic, normoxic gas. Specifically, we determined that exposure to 20% CO2/normoxic gas rapidly induced pressor and bradycardia responses and subsequent increases in anxiety behavior. Locomotor activity was unaffected by the hypercarbic gas exposure, which suggests that this challenge was not anesthetizing or sedating the rats. Hypercarbic gas also increased cellular responses (ie, increased c-Fos expression) in the ORX neurons in the DMH/PeF, but not in the adjacent LH. In support of a role of ORX, systemically pre-treating rats with a panicolytic dose (Johnson et al, 2010) of an ORX1 receptor antagonist (known to cross the blood–brain barrier; Ishii et al, 2005) before the hypercapnic challenge blocked pressor responses and attenuated anxiety-like behavior responses. Surprisingly, hypercarbic gas-induced bradycardia were unaffected by the ORX1 receptor antagonist. The lack of effect of the ORX1 receptor antagonist in the bradycardia response suggests there is a dissociation of the sympathetic and parasympathetic drives following exposure to high CO2 levels. In a subsequent set of experiments, the ORX 1 receptor antagonist had no effect on hypercapnia-induced respiratory responses in anesthetized rats, and in conscious rats only attenuated respiratory responses to the hypercapnic gas at the offset of the challenge. The subsequent sections discuss potential central nervous system (CNS) targets through which ORX may be regulating the hypercapnia-induced behavioral and physiological responses seen here.
ORX's Role in Hypercapnia-Induced Anxiety-Like Responses
Unconditioned anxiety-like behavioral responses following hypercarbic gas exposure, as seen here, are potentially mobilized from the terminal release of ORX at forebrain targets regulating emotional behavior, such as the bed nucleus of the stria terminalis (BNST). The BNST has increased cellular responses to the hypercarbic gas challenge used here (Johnson et al, 2011), is a critical site for unconditioned anxiety-related stress responses (Walker and Davis, 1997; Davis and Shi, 1999; Walker et al, 2003), and contains extensive orexinergic fibers (Peyron et al, 1998) as well as ORX1 (Hervieu et al, 2001) and ORX2 (Cluderay et al, 2002) receptors. Furthermore, ORX injections into the BNST increases anxiety-like behavior (Truitt et al, 2009), and injecting the SB334867 ORX1 receptor antagonist into the BNST blocks anxiety in an animal model of panic (Johnson et al, 2010).
ORX's Role in Hypercapnia-Induced Pressor Responses
The hypercapnia-induced pressor response is primarily a result of sympathetic mobilization that is mediated by chemoreceptors in the CNS (Elam et al, 1981, Fukuda et al, 1989,; Bakehe et al, 1996; Oikawa et al, 2005). As stated in introduction, ORX neurons are CO2 chemosensitive, and ORX neurons can increase sympathetic outflow. For instance, intracerebroventricular injections of ORX produces tachycardia and hypertension as well as increasing renal sympathetic activity and plasma concentrations of norepinephrine and epinephrine (Shirasaka et al, 1999, 2002). Furthermore, mice lacking the ORX precursor have attenuated cardioexcitatory responses following disinhibition of the DMH (Kayaba et al, 2003), and selective lesions of ORX neurons (utilizing an ORX-saporin technique) reduce conditioned fear-induced tachycardia and hypertension (Furlong et al, 2009). Another line of evidence comes from microinjection studies assessing the role of ORX in sympathetic regions, such as the rostro-ventrolateral medulla (RVLM) that is innervated with ORX-containing fibers and express ORX 1 and 2 receptors (Ferguson and Samson, 2003). Pressor responses, elicited by disinhibition of the DMH region containing ORX neurons, appear to be mediated via the RVLM (Fontes et al, 2001), and injecting ORX directly into the RVLM elicits tachycardia (Chen et al, 2000, Ciriello et al, 2003) and hypertension (Chen et al, 2000; Machado et al, 2002, Ciriello et al, 2003).
Dissociation of Mechanisms Underlying Hypercapnia-Induced Sympathetic and Parasympathetic Responses
We found that, whereas the ORX1 receptor antagonist attenuated the hypertensive response to 20% CO2 inhalation, it had no effect on the robust bradycardic response, suggesting that CO2-mediated bradycardia does not involve an ORX1 receptor-dependent mechanism (we did not rule out ORX2 receptor involvement). This dissociation of hypercapnia-induced sympathetic and parasymapthetic responses by ORX1 receptor antagonist treatment sheds some clarity on existing controversies about the mechanisms involved in hyprecapnia-induced bradycardia response.
It is generally accepted that the effector arm for the hypercapnia-induced bradycardia is mediated via activation of vagal input to the heart (Oikawa et al, 2005). However, there is some dispute as to whether the afferent arm is activated by a baroreceptor or chemoreceptor mechanism. Aotic denervation (baroreceptors located in the aortic arch are critical for rapidly altering HR in response to dramatic changes in blood pressure; Spyer, 1990) or atropine treatment, but not carotid sinus denervation (contains CO2/H+ chemosensory glomus cells; Gonzalez et al, 1992, Peers and Buckler, 1995), blocks hypercarbic gas-induced bradycardia without altering pressor responses (Oikawa et al, 2005). This suggests that the hypercapnia-induced bradycardia is baroreflex mediated. Walker and Brizzee (1990) also showed that baroreceptor denervated rats had no bradycardic response to 10% CO2—but because neither the intact rat nor the baroreceptor denervated rat had any increase in blood pressure in response to the CO2, it is unlikely that the baroreflex was activated in their experiments and suggests that the aortic arch may express CO2/H+ chemoreceptive cells.
There is also good evidence for specific chemoreceptor-mediated parasympathetic activation by acute changes in CO2 in humans. A single breath of 35% CO2 results in a decrease in HR preceding any change in blood pressure, thus arguing against a baroreflex effect (Griez and Van den Hout, 1983; Argyropoulos et al, 2002; Kaye et al, 2004).
Our cellular studies further support a chemoreceptor mechanism. Specifically, we have shown that 20% hypercarbic gas exposure induces robust cellular responses (ie, c-Fos expression) in sympathoexcitatory brain regions such as the RVLM and medullary raphe, but had little effect on cellular responses in regions of the brain involved in the baroreflex (ie, nucleus of the solitary tract (NTS) or caudal ventrolateral medulla (CVLM) Johnson et al, 2011; also see review, Dampney, 1994). In contrast, intravenous infusions of the sympathomimetic phenylephrine (an α1-adrenoceptor agonist) induces clear activation of baroreceptor pathways (ie, increase in c-Fos induction in the NTS and CVLM; Chan and Sawchenko, 1998). Overall the experimental data clearly suggest that severe hypercapnia-induced bradycardia occurs in response to peripheral chemoreceptor activation linked to subsequent stimulation of vagal nerve activity (see hypothetical illustration in Figure 5). Finally, in the present study, if the hypercapnic-induced bradycardia was driven by the baroreflex, then the ORX1 receptor antagonist (that blocked the pressor response to CO2) should have also blocked the bradycardia response.
The illustration depicts a hypothetical mechanism through which exposure to brief hypercarbic gas induces physiologic and behavioral responses. The left side illustrates how exposing rats ⩽5% CO2 hypercarbic gas increases respiration rate and tidal volume, but not ‘panic-associated’ responses, by interacting with chemoreceptors in the Pre-Bötzinger complex (Pre-Bötz) and raphe pallidus (RPa) that increase phrenic nerve activity that serve to reduce partial pressure of CO2 (PCO2) without mobilizing other components of the ‘panic-like’ response (Akilesh et al, 1997). However, exposing rats to higher concentrations of hypercarbic gas (eg, 20% CO2) depolarizes ORX neurons by interacting with pH/CO2 chemosensitive K+ channels (Williams et al, 2007), and causes subsequent release of ORX at postsynaptic targets in the brain and spinal cord (see green lines with (+) stimulatory symbols and green ORX 1 postsynaptic receptors) to mobilize anxiety-like behavior, hypertension, and increased ventilatory responses. Illustration also shows SB33486 blocking the postsynaptic effects of the ORX at the ORX1 receptor (see dashed red lines and minus symbols) to attenuate all hypercapnia-induced physiological effects (ie, anxiety and hypertension) except bradycardia, which (see Discussion section) is most likely a result of hypercapnia interacting with peripheral chemoreceptors. Other blue lines indicate direct effects of CO2 on other known chemosensitive brain regions such as the LC, DRN, RVLM, IML, RPa and Pre-Botz. BNST, bed nucleus of the stria terminalis; DMH/PeF; dorsomedial/perifornical hypothalamus; DRN, dorsal raphe nucleus; IML, intermediolateral cell column; LC, locus coeruleus; ORX, orexin; ORX1R, orexin 1 receptor; PBN, parabrachial nucleus; Pre-Bot, pre-Bötzinger complex; RPa, raphe pallidus; RVLM, rostroventrolateral medulla; RTN, retrotrapezoid nucleus.
Putative Mechanism of Hypercapnia Activation of ORX Neurons
Hypercapnia-induced increases in c-Fos expression in the ORX neurons could be secondary to altered synaptic input following activation of peripheral or central chemoreceptor sites, or it could be a direct effect of altered local PCO2/H+ on the neurons themselves. In support of the latter explanation, previous evidence has shown that DMH/PeF regions respond directly to subtle increases in local CO2/H+ concentrations (Dillon and Waldrop, 1992), which could account for hypercapnic challenge-induced c-Fos expression in ORX neurons. Another recent study determined ORX neurons are highly sensitive to changes in local concentration of CO2/H+ concentrations, which is most likely through CO2/H+-induced closure of leak like K+ channels on ORX neurons (Williams et al, 2007). The ORX neuronal response to acute 20% hypercarbic gas exposure in the present study was restricted to the DMH/PeF region. This response is consistent with data from a recent article where 3-h exposure to 10% hypercarbic/normoxic gas also increased c-Fos expression in ORX neurons in the DMH, but not LH region (Sunanaga et al, 2009). The reason for this pattern may be due to: (1) pH-sensitive K+ channels being more prevalent in the DMH/PeF rather than LH (Talley et al, 2001); (2) owing to the acidosis being restricted to the DMH/PeF, which is more proximal to cerebrospinal fluid (CSF) in the third ventricle; or (3) neuronal afferents that mobilize ORX neurons specifically in the DMH/PeF region.
ORX 1 Receptor's Role in Hypercapnia-Induced Respiratory Responses
In the present studies, hypercapnia exposure increased respiration rate in conscious rats. However, pre-treating rats with an ORX1 receptor antagonist only altered the respiratory response to the hypercapnia challenge following the offset of the CO2 infusion. In conscious rats, hypercapnia exposure causes an increase in the respiratory rate from ∼120 to ∼150 breaths per min (b.p.m.) that became more paced during the hypercapnia exposure when the rat had less locomotor activity. Then at the offset of the hypercapnia, the respiratory rate increased from ∼150 to >200 b.p.m., which coincided with an increase in sniffing and locomotor behavior. This suggests that the ORX1 receptor antagonist is not directly altering respiratory drive at the dose and route used here, but rather the behavioral arousal post hypercapnia exposure.
Others have shown that the ORX's effects on hypercapnia-induced respiration may be state dependent. For instance, ORX knockout mice have blunted respiratory responses to 5 and 10% CO2 exposure during wakefulness, but not during sleep states (Kuwaki et al, 2008). Furthermore, systemic injections of the dual ORX antagonist almorexant decreased respiration responses to exposure to 7% CO2, but only during wakefulness (Nattie and Li, 2010). Another consideration is that the studies conducted here were done during the inactive period when CSF levels of ORX are lowest during the 24-h period (Desarnaud et al, 2004), which could explain the lack of effect of the ORX1 receptor antagonist on the respiratory response during the hypercapnia challenge. Thus, ORX's regulation of hypercapnia-induced respiratory responses appears to be most potent during conscious wake period and also during periods of heightened behavioral activity.
Technical Considerations
It is important to clarify that infusing 20% CO2 hypercapnic gas into test cages leads to gradual increases in CO2 that only reach 20% at the end of the 5-min infusion, then rapidly decrease when the atmospheric air is infused. Yet, even then this challenge represents a high concentration of CO2 that elucidates mechanisms, but leads to PaCO2 changes that may fall outside of physiological range under even extreme COPD states.
Conclusions and Clinical Considerations
Episodes of hypercapnia that are associated with COPD, bronchitis, or asthma, lead to severe anxiety and sympathetic arousal, both of which can make management of these patients difficult. Currently anxiety associated with conditions such as COPD is treated with fast-acting benzodiazepine drugs. However, this is not safe because of significant respiratory depression and other peripheral side effects. Recently, COPD was modeled in rats by exposing them to chronic cigarette smoke (1 h, twice/day over 12 weeks) (Liu et al, 2010). By week 12, the COPD rats compared with control rats, had: (1) COPD-associated lung pathology (ie, coalesced alveoli and thickened bronchiolar walls); (2) >100% increase in hypothalamic and medullary ORX-A protein expression; and (3) heightened phrenic nerve responses to ORX-A injections into the pre-Bötzinger complex. Furthermore, in a recent clinical study, ORX-A, which crosses blood–brain barrier easily (Kastin and Akerstrom, 1999), was increased threefold in the plasma of patients with COPD and hypercapnic respiratory failure, compared with controls (Zhu et al, 2011). Our results suggest that the ORX system may have an important role in these responses to hypercapnia, and that antagonizing the ORX1 receptor may treat anxiety associated with hypercapnia without causing significant respiratory depression. Furthermore, ORX1 receptor antagonists also reduce hypertensive responses because of hypercapnia, which may also be exacerbated by the use of sympathomimetics and bronchodilators in COPD. Doses of the ORX1 receptor antagonist used here were anxiolytic and panicolytic without inducing somnolence. We have also previously shown that the dose of the ORX1 receptor antagonist used here does not alter baseline MAP, HR, or locomotion in untreated control rats (Johnson et al, 2010). A caveat is that we did not look at long-term effects of repeated use of the ORX1 receptor antagonist, which may alter wakefulness and baseline cardiorespiratory activity. Thus, the ORX system may also be an important target in future management of this and other hypercapnic conditions.
On a final note, the data presented here may also be relevant to patients with panic disorder. Mild hypercapnia (5–7% CO2), which is normally below the threshold to provokes panic and anxiety responses, elicits panic attacks in the majority of patients with panic disorder compared with few healthy controls (Gorman et al, 1984, 1988; Goetz et al, 2001). This led Klein (1993) to propose that the ‘suffocation’/CO2 monitors in the brain of some patient with panic disorder are hypersensitive to CO2 and lead to panic responses to slight changes in ambient CO2 In a recent review, Freire et al (2010) discuss supporting evidence for panic vulnerability to CO2 in subtypes of panic disorder with comorbid respiratory symptoms. Further preclinical and clinical studies will need to further confirm this phenomenon and determine whether the ORX system may have a role.
References
Akilesh MR, Kamper M, Li A, Nattie EE (1997). Effects of unilateral lesions of retrotrapezoid nucleus on breathing in awake rats. J Appl Physiol 82: 469–479.
Argyropoulos SV, Bailey JE, Hood SD, Kendrick AH, Rich AS, Laszlo G et al (2002). Inhalation of 35% CO(2) results in activation of the HPA axis in healthy volunteers. Psychoneuroendocrinology 27: 715–729.
Bailey JE, Argyropoulos SV, Kendrick AH, Nutt DJ (2005). Behavioral and cardiovascular effects of 7.5% CO2 in human volunteers. Depress Anxiety 21: 18–25.
Bakehe M, Hedner J, Dang T, Chambille B, Gaultier CL, Escourrou P (1996). Role of the autonomic nervous system in the acute blood pressure elevation during repetitive hypoxic and hypercapnic breathing in rats. Blood Press 5: 371–375.
Chan RK, Sawchenko PE (1998). Organization and transmitter specificity of medullary neurons activated by sustained hypertension: implications for understanding baroreceptor reflex circuitry. J Neurosci 18: 371–387.
Chen CT, Hwang LL, Chang JK, Dun NJ (2000). Pressor effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats. Am J Physiol Regul Integr Comp Physiol 278: R692–R697.
Ciriello J, Li Z, de Oliveira CV (2003). Cardioacceleratory responses to hypocretin-1 injections into rostral ventromedial medulla. Brain Res 991: 84–95.
Cluderay JE, Harrison DC, Hervieu GJ (2002). Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul Pept 104: 131–144.
Cuccheddu T, Floris S, Serra M, Porceddu ML, Sanna E, Biggio G (1995). Proconflict effect of carbon dioxide inhalation in rats. Life Sci 56: L321–L324.
Dampney RA (1994). Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364.
Davis M, Shi C (1999). The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann NY Acad Sci 877: 281–291.
Deng BS, Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T (2007). Contribution of orexin in hypercapnic chemoreflex: evidence from genetic and pharmacological disruption and supplementation studies in mice. J Appl Physiol 103: 1772–1779.
Desarnaud F, Murillo-Rodriguez E, Lin L, Xu M, Gerashchenko D, Shiromani SN et al (2004). The diurnal rhythm of hypocretin in young and old F34 rats. Sleep 27: 851–856.
Dillon GH, Waldrop TG (1992). In vitro responses of caudal hypothalamic neurons to hypoxia and hypercapnia. Neuroscience 51: 941–950.
Elam M, Yao T, Thoren P, Svensson TH (1981). Hypercapnia and hypoxia: chemoreceptor-mediated control of locus coeruleus neurons and splanchnic, sympathetic nerves. Brain Res 222: 373–381.
Ferguson AV, Samson WK (2003). The orexin/hypocretin system: a critical regulator of neuroendocrine and autonomic function. Front Neuroendocrinol 24: 141–150.
Fontes MA, Tagawa T, Polson JW, Cavanagh SJ, Dampney RA (2001). Descending pathways mediating cardiovascular response from dorsomedial hypothalamic nucleus. Am J Physiol Heart Circ Physiol 280: H2891–H2901.
Forster HV, Smith CA (2010). Contributions of central and peripheral chemoreceptors to the ventilatory response to Co2/H+. J Appl Physiol 108: 989–994.
Freire RC, Perna G, Nardi AE (2010). Panic disorder respiratory subtype: psychopathology, laboratory challenge tests, and response to treatment. Harv Rev Psychiatry 18: 220–229.
Fukuda Y, Sato A, Suzuki A, Trzebski A (1989). Autonomic nerve and cardiovascular responses to changing blood oxygen and carbon dioxide levels in the rat. J Auton Nerv Syst 28: 61–74.
Furlong TM, Vianna DM, Liu L, Carrive P (2009). Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur J Neurosci 30: 1603–1614.
Goetz RR, Klein DF, Papp LA, Martinez JM, Gorman JM (2001). Acute panic inventory symptoms during CO(2) inhalation and room-air hyperventilation among panic disorder patients and normal controls. Depress Anxiety 14: 123–136.
Gonzalez C, Almaraz L, Obeso A, Rigual R (1992). Oxygen and acid chemoreception in the carotid body chemoreceptors. Trends Neurosci 15: 146–153.
Gorman JM, Askanazi J, Liebowitz MR, Fyer AJ, Stein J, Kinney JM et al (1984). Response to hyperventilation in a group of patients with panic disorder. Am J Psychiatry 141: 857–861.
Gorman JM, Fyer MR, Goetz R, Askanazi J, Liebowitz MR, Fyer AJ et al (1988). Ventilatory physiology of patients with panic disorder. Arch Gen Psychiatry 45: 31–39.
Griez E, Van den Hout MA (1983). Carbon dioxide and anxiety: cardiovascular effects of a single inhalation. J Behav Ther Exp Psychiatry 14: 297–304.
Guyenet PG, Stornetta RL, Abbott SB, Depuy SD, Fortuna MG, Kanbar R (2010). Central CO2 chemoreception and integrated neural mechanisms of cardiovascular and respiratory control. J Appl Physiol 108: 995–1002.
Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA (2001). Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience 103: 777–797.
Irwin SA, Iglewicz A (2010). Oral ketamine for the rapid treatment of depression and anxiety in patients receiving hospice care. J Palliat Med 13: 903–908.
Ishii Y, Blundell JE, Halford JC, Upton N, Porter R, Johns A et al (2005). Anorexia and weight loss in male rats 24 h following single dose treatment with orexin-1 receptor antagonist SB-334867. Behav Brain Res 157: 331–341.
Johnson PL, Fitz SD, Hollis JH, Moratalla R, Lightman SL, Shekhar A et al (2011). Induction of c-Fos in ‘panic/defence’-related brain circuits following brief hypercarbic gas exposure. J Psychopharmacol 25: 26–36.
Johnson PL, Hollis JH, Moratalla R, Lightman SL, Lowry CA (2005). Acute hypercarbic gas exposure reveals functionally distinct subpopulations of serotonergic neurons in rats. J Psychopharmacol 19: 327–341.
Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S et al (2010). A key role for orexin in panic anxiety. Nat Med 16: 111–115.
Kabir MM, Beig MI, Baumert M, Trombini M, Mastorci F, Sgoifo A et al (2010). Respiratory pattern in awake rats: effects of motor activity and of alerting stimuli. Physiol Behav 101: 22–31.
Kastin AJ, Akerstrom V (1999). Orexin A but not orexin B rapidly enters brain from blood by simple diffusion. J Pharmacol Exp Ther 289: 219–223.
Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I et al (2003). Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol 285: R581–R593.
Kaye J, Buchanan F, Kendrick A, Johnson P, Lowry C, Bailey J et al (2004). Acute carbon dioxide exposure in healthy adults: evaluation of a novel means of investigating the stress response. J Neuroendocrinol 16: 1–9.
Klein DF (1993). False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Arch Gen Psychiatry 50: 306–317.
Kuwaki T, Zhang W, Nakamura A, Deng BS (2008). Emotional and state-dependent modification of cardiorespiratory function: role of orexinergic neurons. Auton Neurosci 142: 11–16.
Liu Z, Song N, Geng W, Jin W, Li L, Cao Y et al (2010). Orexin-a and respiration in a rat model of smoke-induced chronic obstructive pulmonary disease. Clin Exp Pharmacol Physiol 37: 963–968.
Machado BH, Bonagamba LG, Dun SL, Kwok EH, Dun NJ (2002). Pressor response to microinjection of orexin/hypocretin into rostral ventrolateral medulla of awake rats. Regul Pept 104: 75–81.
Marotta SF, Sithichoke N, Garcy AM, Yu M (1976). Adrenocortical responses of rats to acute hypoxic and hypercapnic stresses after treatment with aminergic agents. Neuroendocrinology 20: 182–192.
Nattie E, Li A (2010). Central chemoreception in wakefulness and sleep: evidence for a distributed network and a role for orexin. J Appl Physiol 108: 1417–1424.
Oikawa S, Hirakawa H, Kusakabe T, Nakashima Y, Hayashida Y (2005). Autonomic cardiovascular responses to hypercapnia in conscious rats: the roles of the chemo- and baroreceptors. Auton Neurosci 117: 105–114.
Paxinos G, Watson C (1997) The Rat Brain Stereotaxic Coordinates. Academic Press: San Diego.
Peers C, Buckler KJ (1995). Transduction of chemostimuli by the type I carotid body cell. J Membrane Biol 144: 1–9.
Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG et al (1998). Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18: 9996–10015.
Sakurai T (2007). The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci 8: 171–181.
Samuels BC, Zaretsky DV, DiMicco JA (2004). Dorsomedial hypothalamic sites where disinhibition evokes tachycardia correlate with location of raphe-projecting neurons. Am J Physiol Regul Integr Comp Physiol 287: R472–R478.
Shekhar A, Keim SR, Simon JR, McBride WJ (1996). Dorsomedial hypothalamic GABA dysfunction produces physiological arousal following sodium lactate infusions. Pharmacol Biochem Behav 55: 249–256.
Shirasaka T, Kunitake T, Takasaki M, Kannan H (2002). Neuronal effects of orexins: relevant to sympathetic and cardiovascular functions. Regul Pept 104: 91–95.
Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H (1999). Sympathetic and cardiovascular actions of orexins in conscious rats. Am J Physiol 277: R1780–R1785.
Sithichoke N, Malasanos LJ, Marotta SF (1978). Cholinergic influences on hypothalamic-pituitary-adrenocortical activity of stressed rats: an approach utilizing choline deficient diets. Acta Endocrinol (Copenh) 89: 737–743.
Sithichoke N, Marotta SF (1978). Cholinergic influences on hypothalamic-pituitary-adrenocortical activity of stressed rats: an approach utilizing agonists and antagonists. Acta Endocrinol (Copenh) 89: 726–736.
Spyer KM (1990) The central nervous organization of reflex circuitry control. In: Loewy AD (ed). Central Regulation of Autonomic Function. Oxford: New York, pp 168–188.
Sunanaga J, Deng BS, Zhang W, Kanmura Y, Kuwaki T (2009). CO2 activates orexin-containing neurons in mice. Respir Physiol Neurobiol 166: 184–186.
Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA (2001). Cns distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci 21: 7491–7505.
Truitt W, Johnson PL, Dietrich A, Kelley PE, Fitz SD, Shekhar A (2009). Anxiety-like responses induced by orexin A in the BNST are attenuated by NMDA antagonism. Soc for Neurosci (abstract).
Walker BR (1987). Cardiovascular effect of V1 vasopressinergic blockade during acute hypercapnia in conscious rats. Am J Physiol 252: R127–R133.
Walker BR, Brizzee BL (1990). Cardiovascular responses to hypoxia and hypercapnia in barodenervated rats. J Appl Physiol 68: 678–686.
Walker DL, Davis M (1997). Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci 17: 9375–9383.
Walker DL, Toufexis DJ, Davis M (2003). Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol 463: 199–216.
Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D (2007). Control of hypothalamic orexin neurons by acid and CO2 . Proc Natl Acad Sci USA 104: 10685–10690.
Zhu LY, Summah H, Jiang HN, Qu JM (2011). Plasma orexin-a levels in COPD patients with hypercapnic respiratory failure. Mediators Inflamm 2011: 754847.
Acknowledgements
We would like to thank Scott Barton from the University of Notre Dame for assistance on behavioral studies and Amy Dietrich for technical assistance in immunohistochemistry experiment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that this work was supported with grants from Indiana CTSI (UL1 RR025761 to AS) Project Development Award, NIH Student LRP, National Alliance for Schizophrenia and Depression Young Investigators Award to PLJ; Indiana CTSI Project Development Team pilot grant (UL1 RR025761), R01 MH52619 to AS. Within the last 3 years, AS and PLJ received research grants from Johnson and Johnson and Eli Lilly for conducting preclinical studies that are unrelated to the present paper. PLJ and AS also have a patent filed for the use of orexin receptor antagonists in the treatment of anxiety. In the past 3 years, CAL has received compensation from Enlight Biosciences. The remaining authors (BCF, SDF, and SLL) declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Johnson, P., Samuels, B., Fitz, S. et al. Activation of the Orexin 1 Receptor is a Critical Component of CO2-Mediated Anxiety and Hypertension but not Bradycardia. Neuropsychopharmacol 37, 1911–1922 (2012). https://doi.org/10.1038/npp.2012.38
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2012.38
Keywords
This article is cited by
-
Identification of a novel perifornical-hypothalamic-area-projecting serotonergic system that inhibits innate panic and conditioned fear responses
Translational Psychiatry (2024)
-
CO2 reactivity as a biomarker of exposure-based therapy non-response: study protocol
BMC Psychiatry (2022)
-
Acute orexin antagonism selectively modulates anticipatory anxiety in humans: implications for addiction and anxiety
Translational Psychiatry (2022)
-
Understanding rat emotional responses to CO2
Translational Psychiatry (2020)
-
Disruption of estradiol regulation of orexin neurons: a novel mechanism in excessive ventilatory response to CO2 inhalation in a female rat model of panic disorder
Translational Psychiatry (2020)