Abstract
The magnetism of nanoparticles and thin films of wide-bandgap oxides that include no magnetic cations is an unsolved puzzle1. Progress has been hampered by both the irreproducibility of much of the experimental data, and the lack of any generally accepted theoretical explanation. The characteristic signature is a virtually anhysteretic, temperature-independent magnetization curve that saturates in an applied field that is several orders of magnitude greater than the magnetization. It would seem as if a tiny volume fraction, ≲0.1%, of the samples is magnetic and that the energy scale is unusually high for spin magnetism. Here we investigate the effect of dispersing 4 nm CeO2 nanoparticles with powders of γAl2O3, sugar or latex microspheres. The saturation magnetization, Ms ≍ 60 A m−1 for compact samples, is maximized by 1 wt% lanthanum doping. Dispersing the CeO2 nanopowder reduces its magnetic moment by up to an order of magnitude, and there is a characteristic length scale of order 100 nm for the magnetism to appear in CeO2 nanoparticle clusters. The phenomenon is explained in terms of a giant orbital paramagnetism that appears in coherent mesoscopic domains due to resonant interaction with zero-point fluctuations of the vacuum electromagnetic field. The theory explains the observed temperature-independent magnetization curve and its doping and dispersion dependence, based on a length scale of 300 nm that corresponds to the wavelength of a maximum in the ultraviolet absorption spectrum of the magnetic CeO2 nanoparticles. The coherent domains occupy roughly 10% of the sample volume.
Similar content being viewed by others
Main
There is a class of thin films and nanoparticles of oxides that exhibit ferromagnetic-like magnetization curves, although the materials lack the concentration of ions with unpaired d or f electron spins required to generate the exchange interactions needed for high-temperature ferromagnetism1. This is forcing a re-evaluation of the meaning of magnetic saturation in systems that exhibit very little hysteresis. Research in this area has been plagued by a shortage of reproducible experimental data, so there is need for an easily synthesized ‘fruitfly’ system that reliably exhibits stable anomalous magnetism for which no extraneous explanation is possible. The much-studied dilute Co-doped ZnO thin films2 were problematic because metallic cobalt nanoparticles, difficult to detect in ZnO films1,2,3,4, are ferromagnetic with a high Curie temperature.
The reports of magnetism in these oxide systems have shown that a 3d dopant is unnecessary, and even when 3d ions are present they do not necessarily order magnetically5,6,7,8. The magnetism is somehow related to defects; candidates include cation9 or oxygen10 vacancies (F-centres). Sundaresan et al. 11 have suggested that weak room-temperature magnetism could actually be a general feature of oxide nanoparticles. A significant observation was that the magnetism appearing in undoped 10 nm ZnO nanoparticles depends on how they are capped with different molecules, which alter the electronic structure of the surface12.
A promising candidate system is CeO2, where nanoparticles produced in different laboratories often exhibit weak ‘ferromagnetic-like’ behaviour at room temperature. A selection of data is presented in Table 1. Although values of saturation magnetization Ms are very small and vary widely, the saturation field H0 obtained by extrapolating the initial susceptibility to saturation is roughly 1,000 times greater and lies in a narrower range of 40–120 kA m−1. The ratio NeffMs/H0 with an effective demagnetizing factor Neff ≍ 0.3 is a measure of the magnetic volume fraction f in a ferromagnetic system where the approach to saturation is governed by dipolar interactions1. In that case f would be of the order of 0.1%.
We synthesized many small batches (∼4 mg) of nanocrystalline CeO2 powder using as precursor either high-purity (99.999%) Ce(NO3)3 ⋅ 6H2O, or reagent-grade (99%) with La as the main impurity. The nanoparticles are well crystallized, and only 2r = 4 nm in diameter (Fig. 1a, b). Magnetization curves of nanoparticles produced from the two precursors are compared in Fig. 2a. The pure sample shows a practically linear paramagnetic response, but the impure sample exhibits a superposed ferromagnetic-like curve with no evidence of hysteresis. The averaged specific magnetization σs for 16 samples is 0.011 ± 0.006 A m2 kg−1, corresponding to an average moment per Ce ion of 3 × 10−4 μB and an average saturation magnetization Ms = 84 A m−1. These numbers can be misleading. There is evidence that the moment is associated with defects13,14,15 or with the nanoparticle surface12,16 rather than cerium ions distributed homogeneously throughout the volume, so it is better to think of a few tenths of a Bohr magneton as the average moment per particle. The content of Fe, Co and Ni impurities in the 99% CeO2 nanoparticles—10 ppm in total—is too little to account for the observed moments, because each nanoparticle contains approximately 900 cerium atoms.
a, X-ray diffraction pattern showing the reflections of the fluorite structure, with corresponding particle size broadening. b, Transmission electron micrograph of a few of the particles. c, Schematic electronic structure of oxygen-deficient CeO2. d, Ultraviolet–visible absorption spectra of CeO2 micropowders and nanopowders dispersed in 0.1 M H2SO4. The spectra of the magnetic nanoparticles show a peak at 4 eV.
a, Room-temperature magnetization curves of samples prepared from 99.999% and 99% precursors; the saturation magnetization Ms and the saturation field H0 are defined as shown. b, The variation of Ms for nanoparticles produced from the pure precursor with La nitrate addition; magnetization is turned on by La substitution, and it is greatest for a La content of 1%. c, Magnetization curves of a 99% sample at 4 K, 295 K and 380 K. The data are corrected for the diamagnetism of the sample holder and the high-field susceptibility of the sample; the magnetization curves are fitted to equation (2), yielding identical parameters at all temperatures. d, The Curie law susceptibility deduced from the high-field slope. The plot of susceptibility versus inverse temperature in the inset corresponds to just 0.4% of Ce3+ and a paramagnetic Curie temperature of −8 K, assuming a localized moment of peff = 2.54μB.
In fact, it is the lanthanum doping that is responsible for the moment. On doping the pure cerium nitrate precursor with pure lanthanum nitrate, there is a sharp maximum in magnetization for Ce1−xLaxO2 at x = 1.0% and the moment has almost disappeared at x = 10% (Fig. 2b). A similar decline has been observed for Pr doping14. The moments are fairly stable in time, decaying by about a tenth over the course of two years. Moreover, the magnetization curves between 4 K and 380 K superpose after correcting for the high-field slope (Fig. 2c), with little sign of hysteresis at any temperature. The insensitivity of the magnetization to thermal excitations, at least up to 380 K, is evidence that an unusually large energy scale >0.1 eV must be involved.
Like Ce4+, La3+ has a 4f0 configuration, so La substitution in stoichiometric CeO2 would normally create holes at the top of the oxygen 2p band. However, CeO2 is a catalyst renowned for its oxygen vacancies, and the addition of La increases the quantities of both oxygen vacancies and peroxide ions that occur naturally at the CeO2 surface17. The content of localized Ce3+ ions estimated from the Curie-law variation of the susceptibility (Fig. 2d) is only 0.4%. If there are any other Ce3+ electrons, they are delocalized at the bottom of the 4f band as suggested in Fig. 1c. The 2p–4f gap for stoichiometric CeO2 is 3.0–3.5 eV and the 2p–5d/6s gap is 6–8 eV (refs 18,19). Electrons will tend to segregate to the nanoparticle surface, which is conducting for oxygen-deficient CeO2 (refs 19,20). It should be emphasized that Ce3+ compounds or intermetallics rarely order magnetically above 15 K (1.3 meV), and the maximum reported value is 125 K (ref. 21).
In a series of experiments where the CeO2 nanopowder was progressively diluted with powders of different particle size, we used a 15 nm γAl2O3 nanopowder, finely ground icing sugar with an average particle size ∼1 μm and latex microspheres 10 μm in diameter. The surprising effect of dispersion with γAl2O3 is shown in Fig. 3a. When a 4 mg sample of CeO2 is diluted with six times its own volume of diamagnetic γAl2O3, the magnetic moment collapses to just 6% of the original value (Fig. 3). The effect of dilution is to separate clumps of CeO2 nanoparticles ≤100 nm in size. Dilution with finely ground sugar has a similar, if less pronounced effect. The moment there falls by 50% on diluting with 30 times the volume of sugar, which is less effective than γAl2O3 at dispersing the CeO2 particles, but has the advantage that some of the CeO2 can be recovered by dissolving the sugar in water. The specific magnetization for the recovered CeO2 is double that at the outset. Large, 10 μm latex microspheres are least effective; the moment is reduced by 15% for a 20-fold volume dilution. The aggregates of CeO2 coexisting with the microspheres are about 500 nm in size, and sometimes envelop them (Fig. 3d).
a, Magnetization curves for a 4 mg sample of 4 nm CeO2 nanopowder diluted with 15 nm γAl2O3. b, Relative magnetization as a function of dilution of 4 mg of 4 nm CeO2 by weight with 15 nm γAl2O3, 1 μm sugar or 10 μm latex microspheres. Error bars represent standard deviations of measurements on multiple batches. c, Electron micrograph, showing how the dilution with γAl2O3 breaks the CeO2 down into ∼100 nm clumps, which destroys the moment. d, The coating of a 10 μm latex microsphere by CeO2 nanoparticles.
Together, these experiments establish that the magnetism of CeO2 depends critically on the mesoscale disposition of the nanoparticles, as well as doping, which is probably why there is so much variability in the data of Table 1. We conclude that there is a characteristic length scale for the appearance of magnetism, which is of order 100 nm. Previously, it had been reported that the moment of 6 nm nanoparticles of ZnO doped with 0.93% Ni appeared only in reaction-limited aggregates about 400 nm in size22, and that the moment in 7 nm CeO2 powder was modified on sintering13. The magnetism is not simply an intrinsic property governed by atomic-scale defects within the particles. The extent and topology of the surface of contiguous particles is a critical factor.
Until now, a plausible model for the high-temperature magnetism of CeO2 nanoparticles has been Stoner ferromagnetism with a spin-split band associated with conducting surface states23. Furthermore, if the band is half-metallic, spin-wave excitations are suppressed, and a high Curie temperature could be envisaged24. The problem is to understand how, when we break up the CeO2 nanoparticle sample into 100 nm clumps, we can lose the magnetism. Stoner splitting of a 4f band or a defect band, of order a few tenths of an electron volt, will not change appreciably when the sample is divided up. The closest analogy in the conventional paradigm is the stabilization of magnetic order in clusters of superparamagnetic nanoparticles by dipole–dipole interactions25. The magnetite particle chains in magnetotactic bacteria are a good example. Contiguous particles with a magnetization of order 0.5 MA m−1 and a moment of order 1,000μB can provide a dipole interaction energy that exceeds room temperature. However, the average moment of a CeO2 nanoparticle is three or four orders of magnitude too small for this explanation to work.
It seems that a radically new approach is required. We propose that the magnetic saturation is not related to collective spin ferromagnetism but to giant orbital paramagnetism26 associated with the collective response of electrons in coherent domains to an applied magnetic field. Our starting point is a new model27, which showed that when zero-point fluctuations of the vacuum electromagnetic field interact with an ensemble of two-level atoms, it is possible for coherent mesoscopic domains to emerge. This can take place at room temperature in quasi-two-dimensional systems, with a large surface/volume ratio. No resonant cavity is required. The size of the coherent domains is determined by the wavelength corresponding to an electronic excitation ℏω between the ground state and the excited state of the two-level atoms. The excited state lies at an energy ɛ below the ionization threshold, as illustrated in Fig. 4a. The interaction of the N electrons in a coherent domain of size λ ≍ 2πc/ω with the vacuum field leads to stabilization of the ground state and destabilization of the excited state each by an energy G2ℏω, where G is calculated to be ≍0.1 (ref. 27). The model is parameterized in terms of N, ω and ɛ, and the stability condition is kBT < G2ℏω < ɛ.
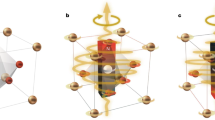
a, Single-atom electronic excitations related to impurities or defects in CeO2, showing the effect of coherent interaction with the vacuum electromagnetic field and the consequent influence of a magnetic field on the ground and excited states. b, A sample of CeO2 where a fraction f of the volume is occupied by coherent domains of dimension λ = 2πc/ω (shaded grey). c, An orbital current in a coherent domain formed from a clump of CeO2 nanoparticles.
In the coherent ground state, the electrons have a common induced oscillation frequency G2ω and a corresponding moment (see Supplementary Information)

that is set by the size of the orbit, where l is the orbital quantum number of the electronic ground state and μB is the Bohr magneton. In the presence of the time-varying vacuum electromagnetic field, the effect of a static magnetic field on the coherent domain is to produce a modified coherent ground state, inducing a paramagnetic orbital moment in the domain that is nonlinear in B and proportional to sin 2αm, where αm is a mixing angle (see Supplementary Information). The magnetization curve has the form

where x = CB ≍ GNμcB/ℏω. This function differs only slightly from the empirical M = Ms tanh y function often used to fit magnetization curves23, but it follows directly from theory. Fits of equation (2) to the curves in Fig. 2c at 4 K, 295 K and 380 K give very similar fit parameters C = 9.4 ± 0.7 T−1 and Ms = 58 ± 1 A m−1.
The length scale in the problem is set by a characteristic excitation frequency ω of CeO2, which is resonant with the zero-point vacuum fluctuations. The corresponding wavelength is λ = 2πc/ω, and the volume of the coherent domain is vc ≍ (π/6)λ3. In Fig. 1d there is a prominent absorption at λ = 300 nm in the ultraviolet spectrum of the magnetic nanoparticles. The corresponding frequency of the electronic transition is ω = 6.3 × 1015 s−1, and the photon energy is ℏω = 4.1 eV. No real photons of this energy are emitted or absorbed according to the theory27; it is the zero-point energy that owing to its time dependence can mix states that differ in energy by ℏω to produce a modulated collective response frequency for all N electrons in a coherent domain.
By fitting the magnetization curve to equation (2), we can deduce the volume fraction fc of the sample that is composed of coherent domains, and estimate their magnetic moment Nμc. Dividing the saturation magnetization Ms ≍ fcGNμc/2vc, by C ≍ GNμc/ℏω, we obtain Ms/C = fcℏω/2vc. With the experimental value of Ms/C and G ≍ 0.1, we find fc = 28% and the coherent domain moment Nμc = 6.6 × 106μB. Identifying N with the number of La dopant atoms in a coherent domain (2.4 × 106), the coherent moment per dopant μc = 2.8μB.
The orbital moment expected from equation (1) depends on G, the orbital quantum number of the ground state and the ionization energy ɛ of the excited state. Taking G ≍ 0.1 and l = 3, identifying the transition that becomes very prominent in the magnetic nanoparticles (Fig. 1d) as a 4f–5d transition, the ionization energy ɛ ≍ 0.1 eV. The values of G and N are in accord with the values anticipated for quasi-two-dimensional coherent domains in the model27 (G ≍ 0.1 and N ≍ 106 for ℏω = 4.1 eV). The model of ref. 27 was simplified, and took no account of the spin of the electrons. The influence of spin–orbit coupling and taking account of Fermi–Dirac statistics in these dilute electronic systems will not modify the semiquantitative agreement between the theory and our experiments. Generally, it will be possible to estimate the size λ of the coherent domains in such systems by fitting the magnetization curve to equation (2) to determine C/Ms and then using the following expression that follows from ℏω = 2πℏc/λ, Ms/C = fcℏω/2vc and vc = πλ3/6

In summary, giant orbital moments related to zero-point fluctuations of the vacuum electromagnetic field can resolve a long-standing problem in magnetism. The theory accounts for the temperature independence of the magnetization curve, and the characteristic length scale of order 100 nm required for the appearance of the magnetically induced orbital currents in coherent domains. The saturation magnetization Ms and the parameter C in equation (2), which are easily obtained by fitting the magnetization curve, determine the size of the coherent domains through equation (3) and the value of their giant orbital moments, assuming G ≍ 0.1. The data on all our magnetic samples, and almost all the other data in Table 1, are consistent with λ ≍ 300 nm, and coherent volume fractions of 1–80%.
Giant orbital paramagnetism is a new observable consequence of zero-point electromagnetic energy—it is the first such magnetic effect. It occurs in mesoscopic quasi-two-dimensional matter where the active sites are dilute and the effects of Fermi–Dirac statistics can be neglected. Spin–orbit interaction is expected to stabilize the coherent state. It is anticipated that the present study will lead further investigations of measurable consequences of resonant zero-point fluctuations not only in magnetic systems such as gold nanoparticles28, but in other areas of condensed matter, whether physics, chemistry or biology. Some candidate systems are nanobubbles, the water/cell interface and concentrated ionic solutions.
Methods
The CeO2 nanoparticles were synthesized by homogeneous precipitation23from 10 mM Ce(NO3)3 ⋅ 6H2O solutions by dropwise addition of 0.1 M NaOH (99.99% purity). Then 0.45 ml (1/10 the volume of Ce(NO3)3 ⋅ 6H2O solution) of 0.5 M polyethylene glycol (PEG) with molecular weight 1,500 is added to help separate the nanoparticles during formation; they are well crystallized, but only about 4 nm in size (Fig. 1). Magnetization was measured on 4 mg samples of CeO2 nanoparticles using a 5 T Quantum Design SQUID magnetometer. Powders were contained in gelcap sample holders, which produce a linear diamagnetic response, mounted in a plastic straw. All isothermal magnetization curves, but not the thermal scans, have been corrected for the linear response.
References
Coey, J. M. D. Handbook of Spin Transport and Magnetism Ch. 20, 405–426 (CRC Press, 2012).
Pan, F., Song, C., Liu, X. J., Yang, Y. C. & Zeng, F. Ferromagnetism and possible application in spintronics of transition-metal doped ZnO films. Mater. Sci. Eng. R 62, 1–35 (2008).
Park, J. H. et al. Co metal clustering as the origin of ferromagnetism in Co-doped ZnO thin films. Appl. Phys. Lett. 84, 1338–1340 (2004).
Dorneles, L. S. et al. Magnetic and structural properties of Co-doped ZnO thin films. J. Magn. Magn. Mater. 310, 2087–2088 (2007).
Barla, A. et al. Paramagnetism of the Co sublattice in ferromagnetic Zn1−xCoxO films. Phys. Rev. B 76, 125201 (2007).
Tietze, T. et al. XMCD studies on Co and Li doped ZnO magnetic semiconductors. New J. Phys. 10, 055009 (2008).
Venkatesan, M. et al. Room-temperature ferromagnetism in Mn- and Fe-doped indium tin oxide thin films. J. Appl. Phys. 103, 07D135 (2008).
Coey, J. M. D., Gunning, R. D., Venkatesan, M., Stamenov, P. & Paul, K. Magnetism in defect-ridden oxides. New J. Phys. 12, 053025 (2010).
Elfimov, I. S., Yunoki, S. & Sawatzky, G. A. Possible path to a new class of ferromagnetic and half-metallic ferromagnetic materials. Phys. Rev. Lett. 89, 216403 (2002).
Banerjee, S., Mandal, M., Gayathri, N. & Sardar, M. Enhancement of ferromagnetism upon thermal annealing in pure ZnO. Appl. Phys. Lett. 91, 182501 (2007).
Sundaresan, A. et al. Ferromagnetism as a universal feature of nanoparticles of otherwise nonmagnetic oxides. Phys. Rev. B 74, 161306(R) (2006).
Chaboy, J. et al. Evidence of intrinsic magnetism in capped ZnO nanoparticles. Phys. Rev. B 82, 064411 (2010).
Fernandez, V. et al. Ferromagnetism induced by oxygen and cerium vacancies above the percolation limit in CeO2 . J. Phys. Condens. Matter 22, 216004 (2010).
Paunovic, N. et al. Suppression of inherent ferromagnetism in Pr-doped CeO2 nanocrystals. Nanoscale 4, 5469–5476 (2012).
Chen, S. Y. et al. Concentration dependence of oxygen vacancy on the magnetism of CeO2 nanoparticles. J. Phys. Chem. C 116, 8707–8713 (2012).
Sundaresan, A. & Rao, C. N. R. Ferromagnetism as a universal feature of inorganic nanoparticles. Nano Today 4, 96–106 (2009).
Keating, P. R. L., Scanlon, D. O. & Watson, G. W. The nature of oxygen states on the surfaces of CeO2 & La-doped CeO2 . Chem. Phys. Lett. 608, 239–243 (2014).
Xiao, X., Jerratsch, J. F., Nilius, N. & Freund, H. J. Probing the 4f states of ceria by tunelling spectroscopy. Phys. Chem. Chem. Phys. 13, 12646–12651 (2011).
Gillen, R., Clark, S. J. & Robertson, J. Nature of the electronic band gap in lanthanide oxides. Phys. Rev. B 87, 125116 (2013).
Popovic, Z. V., Dohcevic-Mitrovic, Z. D., Paunovic, N. & Radovic, M. Evidence of charge delocalization in Ce1−xFex2+(3+)O2−y nanocrystals (x = 0, 0.06, 0.12). Phys. Rev B 85, 014302 (2012).
Givord, F. et al. Ferromagnetism and crystal electric field in the cerium compound CeRh3B2 . J. Phys. Condens. Matter 19, 356208 (2007).
Radovanovic, P. V. & Gamelin, D. R. High-temperature ferromagnetism in Ni2+-doped ZnO aggregates prepared from colloidal dilute magnetic semiconductor quantum dots. Phys Rev. Lett. 91, 157302 (2003).
Ackland, K., Monzon, L. M. A., Venkatesan, M. & Coey, J. M. D. Magnetism of nanostructured CeO2 . IEEE Trans. Magn. 47, 3509–3512 (2011).
Edwards, D. M. & Katsnelson, M. I. High-temperature ferromagnetism of sp electrons in narrow impurity bands: application to CaB6 . J. Phys. C 18, 7209–7225 (2006).
Bedanta, S. & Kleemann, W. Supermagnetism. J. Phys. D 42, 013001 (2009).
Hernando, A., Crespo, P. & Garcia, M. A. Origin of orbital ferromagnetism and giant magnetic anisotropy at the nanoscale. Phys. Rev. Lett. 96, 057206 (2004).
Sen, S., Gupta, K. L. & Coey, J. M. D. Mesoscopic structure formation in condensed matter due to vacuum fluctuations. Phys. Rev. B 92, 155115 (2015).
Nealon, G. L. et al. Magnetism in gold nanoparticles. Nanoscale 4, 5244–5258 (2012).
Liu, Y. et al. Size dependent ferromagnetism in cerium oxide (CeO2) nanostructures independent of oxygen vacancies. J. Phys. Condens. Matter 20, 165201 (2008).
Ge, M. Y. et al. On the origin of ferromagnetism in CeO2 nanocubes. Appl. Phys. Lett. 93, 062505 (2008).
Chen, X. et al. Synthesis and room-temperature ferromagnetism of CeO2 nanocrystals with nonmagnetic Ca2+ doping. Nanotechnology 20, 115606 (2009).
Li, M. et al. Relationship between the surface chemical states and magnetic properties of CeO2 nanoparticles. Appl. Phys. Lett. 94, 152511 (2009).
Chen, S. Y. et al. Oxygen vacancy dependent magnetism of CeO2 nanoparticles prepared by thermal decomposition method. J. Phys. Chem. C 114, 19576–19581 (2010).
Phokha, S. et al. Synthesis, characterization, and magnetic properties of monodisperse CeO2 nanospheres prepared by PVP-assisted hydrothermal method. Nanoscale Res. Lett. 7, 425 (2012).
Acknowledgements
This work was supported by Science Foundation Ireland as part of the NISE project, contract 10/IN1.I3006.
Author information
Authors and Affiliations
Contributions
M.C. conceived the experiment and wrote the paper, K.A. and M.V. carried out the experiments and reduced the data, S.S. developed the theory, and M.C. and S.S. analysed the results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 315 kb)
Rights and permissions
About this article
Cite this article
Coey, M., Ackland, K., Venkatesan, M. et al. Collective magnetic response of CeO2 nanoparticles. Nature Phys 12, 694–699 (2016). https://doi.org/10.1038/nphys3676
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphys3676
This article is cited by
-
Microstructure mediated weak ferromagnetism in La-doped CeO2 nanoparticles
Applied Physics A (2024)
-
The emergence of magnetic ordering at complex oxide interfaces tuned by defects
Nature Communications (2020)
-
Ferromagnetic Bismuth-Substituted CeO2 Nanostructures and Prevalence of Antiferromagnetic Clusters
Journal of Superconductivity and Novel Magnetism (2020)
-
Magnetism in d0 oxides
Nature Materials (2019)
-
Investigation of magnetic properties for Hf4+ substituted CeO2 nanoparticles for spintronic applications
Journal of Materials Science: Materials in Electronics (2018)