Abstract
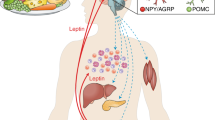
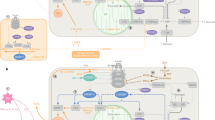
The increasing incidence of obesity in developed nations is an ever-growing challenge to health care, promoting diabetes and other diseases. The hormone leptin, which is derived from adipose tissue, regulates feeding and energy expenditure. Most forms of obesity are associated with diminished responsiveness to the appetite-suppressing effects of leptin. Here we review the mechanisms by which leptin activates intracellular signals, the roles of these signals in leptin action in vivo, and mechanisms that may attenuate leptin signaling, limiting its action in obese individuals. We highlight data regarding the expression of SOCS3 (a potential mediator of leptin resistance) in the arcuate nucleus of the hypothalamus.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Friedman, J.M. & Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 395, 763–770 (1998).
Elmquist, J.K., Elias, C.F. & Saper, C.B. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron 22, 221–232 (1999).
Bates, S.H. & Myers, M.G., Jr. The role of leptin receptor signaling in feeding and neuroendocrine function. Trends Endocrinol. Metab. 14, 447–452 (2003).
Farooqi, I.S. et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Invest. 110, 1093–1103 (2002).
Kamohara, S., Burcelin, R., Halaas, J.L., Friedman, J.M. & Charron, M.J. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature 389, 374–377 (1997).
Burcelin, R. et al. Acute intravenuous leptin infusion increases glucose turnover but not skeletal muscle glucose uptake in ob/ob mice. Diabetes 48, 1264–1269 (1999).
Liu, L. et al. Intracerebroventricular leptin regulates hepatic but not peripheral glucose fluxes. J. Biol. Chem. 273, 31160–31167 (1998).
Schwartz, M.W. et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes 45, 531–535 (1996).
Tartaglia, L.A. The leptin receptor. J. Biol. Chem. 272, 6093–6096 (1997).
Kloek, C. et al. Regulation of Jak kinases by intracellular leptin receptor sequences. J. Biol. Chem. 277, 41547–41555 (2002).
Schwartz, M.W., Woods, S.C., Porte, D., Jr, Seeley, R.J. & Baskin, D.G. Central nervous system control of food intake. Nature 404, 661–671 (2000).
Elias, C.F. et al. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron 23, 775–786 (1999).
Butler, A.A. & Cone, R.D. The melanocortin receptors: lessons from knockout models. Neuropeptides 36, 77–84 (2002).
Cowley, M.A. et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484 (2001).
Morton, G.J. et al. Arcuate nucleus-specific leptin receptor gene therapy attenuates the obesity phenotype of Koletsky (fak/fak) rats. Endocrinology 144, 2016–2024 (2003).
Coppari, R. et al. The hypothalamic arcuate nucleus: A key site for mediating leptin's effects on glucose metabolism and locomotor activity. Cell Metabolism 1, 63–72 (2005).
Balthasar, N. et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42, 983–991 (2004).
Ihle, J.N. & Kerr, I.M. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 11, 69–74 (1995).
White, D.W., Kuropatwinski, K.K., Devos, R., Baumann, H. & Tartaglia, L.A. Leptin receptor (OB-R) signaling. J. Biol. Chem. 272, 4065–4071 (1997).
Banks, A.S., Davis, S.M., Bates, S.H. & Myers, M.G., Jr. Activation of downstream signals by the long form of the leptin receptor. J. Biol. Chem. 275, 14563–14572 (2000).
Bjorbaek, C. et al. Divergent roles of SHP-2 in ERK activation by leptin receptors. J. Biol. Chem. 276, 4747–4755 (2001).
Bjorbaek, C. et al. SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J. Biol. Chem. 275, 40649–40657 (2000).
Munzberg, H., Huo, L., Nillni, E.A., Hollenberg, A.N. & Bjorbaek, C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelano–cortin gene expression by leptin. Endocrinology 144, 2121–2131 (2003).
Bates, S.H. et al. STAT3 signaling is required for leptin regulation of energy balance but not reproduction. Nature 421, 856–859 (2003).
Sasaki, A. et al. CIS3/SOCS3 suppresses erythropoietin signaling by binding the EPO receptor and JAK2. J. Biol. Chem. (2000).
Dunn, S.L. et al. Feedback inhibition of leptin receptor/Jak2 signaling via Tyr1138 of the leptin receptor and SOCS3. Mol. Endocrinol. (2004).
Feng, J. et al. Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol. Cell. Biol. 17, 2497–2501 (1997).
Carpino, N. et al. Identification, cDNA cloning, and targeted deletion of p70, a novel, ubiquitously expressed SH3 domain-containing protein. Mol. Cell. Biol. 22, 7491–7500 (2002).
Argetsinger, L.S. et al. Autophosphorylation of JAK2 on tyrosines 221 and 570 regulates its activity. Mol. Cell. Biol. 24, 4955–4967 (2004).
Feener, E.P., Rosario, F., Dunn, S.L., Stancheva, Z. & Myers, M.G., Jr. Tyrosine phosphorylation of Jak2 in the JH2 domain inhibits cytokine signaling. Mol. Cell. Biol. 24, 4968–4978 (2004).
Kurzer, J.H. et al. Tyrosine 813 is a site of JAK2 autophosphorylation critical for activation of JAK2 by SH2-B beta. Mol. Cell. Biol. 24, 4557–4570 (2004).
Niswender, K.D. et al. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes 52, 227–231 (2003).
Argetsinger, L.S. et al. Growth hormone, interferon-γ, and leukemia inhibitory factor promoted tyrosyl phosphorylation of insulin receptor substrate-1. J. Biol. Chem. 270, 14685–14692 (1995).
Burks, D.J. et al. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature 407, 377–382 (2000).
Niswender, K.D. et al. Intracellular signalling: key enzyme in leptin-induced anorexia. Nature 413, 794–795 (2001).
Zhang, E.E., Chapeau, E., Hagihara, K. & Feng, G.S. Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc. Natl. Acad. Sci. USA 101, 16064–16069 (2004).
Gao, Q. et al. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc. Natl. Acad. Sci. USA 101, 4661–4666 (2004).
Bates, S.H. et al. LRb-STAT3 signaling is required for the neuroendocrine regulation of energy expenditure by leptin. Diabetes 53, 3067–3073 (2004).
Bates, S.H., Kulkarni, R.N., Seifert, M. & Meyers, M.G. Jr. STAT3-independent signaling contributes to regulation of glucose homeostasis by leptin. Cell Metabolism 1, 169–178 (2005).
Roth, J. Diabetes and obesity. Diabetes Metab. Rev. 13, 1–2 (1998).
Licinio, J. et al. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc. Natl. Acad. Sci. USA 101, 4531–4536 (2004).
Oral, E.A. et al. Leptin-replacement therapy for lipodystrophy. N. Engl. J. Med. 346, 570–578 (2002).
Shimomura, I., Hammer, R.E., Ikemoto, S., Brown, M.S. & Goldstein, J.L. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature 401, 73–76 (1999).
Welt, C.K. et al. Recombinant human leptin in women with hypothalamic amenorrhea. N. Engl. J. Med. 351, 987–997 (2004).
Mantzoros, C.S. & Flier, J.S. Editorial: leptin as a therapeutic agent–trials and tribulations. J. Clin. Endocrinol. Metab. 85, 4000–4002 (2000).
Frederich, R.C. et al. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat. Med. 1, 1311–1314 (1995).
Van Heek, M. et al. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J. Clin. Invest. 99, 385–390 (1997).
El Haschimi, K., Pierroz, D.D., Hileman, S.M., Bjorbaek, C. & Flier, J.S. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J. Clin. Invest. 105, 1827–1832 (2000).
Banks, W.A. The many lives of leptin. Peptides 25, 331–338 (2004).
Levin, B.E., Dunn-Meynell, A.A. & Banks, W.A. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R143–R150 (2004).
Krisch, B. & Leonhardt, H. The functional and structural border of the neurohemal region of the median eminence. Cell Tissue Res. 192, 327–339 (1978).
Peruzzo, B. et al. A second look at the barriers of the medial basal hypothalamus. Exp. Brain Res. 132, 10–26 (2000).
Munzberg, H., Flier, J.S. & Bjorbaek, C. Region-specific leptin resistance within the hypothalamus of diet-induced-obese mice. Endocrinology (2004).
Zabolotny, J.M. et al. PTP1B regulates leptin signal transduction in vivo. Dev. Cell 2, 489–495 (2002).
Cheng, A. et al. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev. Cell 2, 497–503 (2002).
Bjorbaek, C., Elmquist, J.K., Frantz, J.D., Shoelson, S.E. & Flier, J.S. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol. Cell 1, 619–625 (1998).
Howard, J.K. et al. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat. Med. (2004).
Mori, H. et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat. Med. (2004).
Tups, A. et al. Photoperiodic regulation of leptin sensitivity in the Siberian hamster, Phodopus sungorus, is reflected in arcuate nucleus SOCS-3 (suppressor of cytokine signaling) gene expression. Endocrinology 145, 1185–1193 (2004).
Emanuelli, B. et al. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J. Biol. Chem. 276, 47944–47949 (2001).
Rui, L., Yuan, M., Frantz, D., Shoelson, S. & White, M.F. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J. Biol. Chem. 277, 42394–42398 (2002).
Ueki, K., Kondo, T. & Kahn, C.R. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol. Cell. Biol. 24, 5434–5446 (2004).
Rossetti, L. Hypothalamic sensing of fatty acids. Nat. Neurosci. 8 579–584 (2005).
Hu, L., Fernstrom, J.D. & Goldsmith, P.C. Exogenous glutamate enhances glutamate receptor subunit expression during selective neuronal injury in the ventral arcuate nucleus of postnatal mice. Neuroendocrinology 68, 77–88 (1998).
Acknowledgements
Supported by grants from the National Institutes of Health and the American Diabetes Association (to M.G.M.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Münzberg, H., Myers, M. Molecular and anatomical determinants of central leptin resistance. Nat Neurosci 8, 566–570 (2005). https://doi.org/10.1038/nn1454
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1454
This article is cited by
-
Effect of intermittent fasting on lipid biokinetics in obese and overweight patients with type 2 diabetes mellitus: prospective observational study
Diabetology & Metabolic Syndrome (2024)
-
The role of immune dysfunction in obesity-associated cancer risk, progression, and metastasis
Cellular and Molecular Life Sciences (2021)
-
Appetite changes reveal depression subgroups with distinct endocrine, metabolic, and immune states
Molecular Psychiatry (2020)
-
Obesity, kidney dysfunction and hypertension: mechanistic links
Nature Reviews Nephrology (2019)
-
Celastrol as a tool for the study of the biological events of metabolic diseases
Science China Chemistry (2019)