Abstract
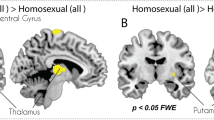
Men are generally more interested in and responsive to visual sexually arousing stimuli than are women. Here we used functional magnetic resonance imaging (fMRI) to show that the amygdala and hypothalamus are more strongly activated in men than in women when viewing identical sexual stimuli. This was true even when women reported greater arousal. Sex differences were specific to the sexual nature of the stimuli, were restricted primarily to limbic regions, and were larger in the left amygdala than the right amygdala. Men and women showed similar activation patterns across multiple brain regions, including ventral striatal regions involved in reward. Our findings indicate that the amygdala mediates sex differences in responsiveness to appetitive and biologically salient stimuli; the human amygdala may also mediate the reportedly greater role of visual stimuli in male sexual behavior, paralleling prior animal findings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Canli, T., Desmond, J.E., Zhao, Z. & Gabrieli, J.D.E. Sex differences in the neural basis of emotional memories. Proc. Natl Acad. Sci. USA 99, 10789–10794 (2002).
Cahill, L. et al. Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiol. Learn. Mem. 75, 1–9 (2001).
Gur, R.C. et al. An fMRI study of sex differences in regional activation to a verbal and spatial task. Brain Lang. 74, 157–170 (2000).
Symons, D. The Evolution of Human Sexuality (Oxford Univ. Press, Oxford, UK, 1979).
Laumann, E.O., Gagnon, J.H., Michael, R.T. & Michaels, S. The Social Organization of Sexuality (University of Chicago Press, Chicago,1994).
Herz, R.S. & Cahill, E.D. Differential use of sensory information in sexual behavior as a function of gender. Hum. Nature 8, 275–286 (1997).
Newman, S.W. The medial extended amygdala in male reproductive behavior: a node in the mammalian social behavior network. Ann. NY Acad. Sci. 877, 242–257 (1999).
Beauregard, M., Levesque, J. & Bourgouin, P.J. Neural correlates of conscious self-regulation of emotion. J. Neurosci. 21, 1–6 (2001).
Redoute, J. et al. Brain processing of visual sexual stimuli in human males. Hum. Brain Mapp. 11, 162–177 (2000).
Arnold, A.P. & Gorski, R.A. Gonadal steroid induction of structural sex differences in the central nervous system. Annu. Rev. Neurosci. 7, 413–442 (1984).
Roselli, C.E., Klosterman, S. & Resko, J.A. Anatomic relationships between aromatase and androgen receptor mRNA expression in the hypothalamus and amygdala of adult male cynomolgus monkeys. J. Comp. Neurol. 439, 208–223 (2001).
Friston, K.J. et al. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 2, 189–210 (1995).
Schmitt, D.P. et al. Universal sex differences in the desire for sexual variety: tests from 52 nations, 6 continents, and 13 islands. J. Pers. Soc. Psychol. 85, 85–104 (2003).
Rauch, S.L. et al. Neural activation during sexual and competitive arousal in healthy men. Psychol. Res. Neuroimag. 91, 1–10 (1999).
Stoleru, S. et al. Neuroanatomical correlates of visually evoked sexual arousal in human males. Arch. Sex. Behav. 28, 1–21 (1999).
Kampe, K.K., Frith, C.D., Dolan, R.J. & Frith, U. Reward value of attractiveness and gaze. Nature 413, 589 (2001).
Gottfried, J.A., O'Doherty, J. & Dolan, R.J. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science 301, 1104–1107 (2003).
Everitt, B.J. Sexual motivation: a neural and behavioral analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci. Biobehav. Rev. 14, 217–232 (1990).
Hamann, S.B., Ely, T.D., Hoffman, J.M. & Kilts, C.D. Ecstasy and agony: activation of the human amygdala in positive and negative emotion. Psychol. Sci. 13, 135–141 (2002).
Garavan, H., Pendergrass, J.C., Ross, T.J., Stein, E.A. & Risinger, R.C. Amygdala response to both positively and negatively valenced stimuli. Neuroreport 12, 2779–2783 (2001).
Karama, S.M. et al. Areas of brain activation in males and females during viewing of erotic film excerpts. Hum. Brain Mapp. 16, 1–13 (2002).
Bradley, M.M., Codispoti, M., Sabatinelli, D. & Lang, P. Emotion and motivation. II: Sex differences in picture processing. Emotion 1, 300–319 (2001).
Anderson, A.K. et al. Dissociated neural representations of intensity and valence in human olfaction. Nat. Neurosci. 6, 196–202 (2003).
Canli, T., Zhao, Z., Brewer, J., Gabrieli, J.D. & Cahill, L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. J. Neurosci. 20, 1–5 (2000).
Everitt, B.J., Cardinal, R.N., Parkinson, J.A. & Robbins, T.W. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann. NY Acad. Sci. 985, 233–250 (2003).
Arana, F.S. et al. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. J. Neurosci. 23, 9632–9638 (2003).
Gallagher, M. The amygdala and associative learning. in The Amygdala: a Functional Analysis edn. 2 (ed. Aggleton, J.P.) 311–330 (Oxford Univ. Press, Oxford, UK, 2001).
Baird, A.D. et al. The amygdala and sexual drive: insights from temporal lobe epilepsy surgery. Ann. Neurol. 55, 87–96 (2004).
Hamann, S.B., Ely, T.D., Grafton, S.T. & Kilts, C.D. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat. Neurosci. 2, 289–293 (1999).
Anderson, A.K. & Phelps, E.A. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature 411, 305–309 (2001).
Adolphs, R., Tranel, D. & Damasio, A.R. The human amygdala in social judgment. Nature 383, 470–473 (1998).
Davis, M. & Whalen, P.J. The amygdala: vigilance and emotion. Mol. Psychiatry 6, 12–34 (2001).
Calder, A.J., Lawrence, A.J. & Young, A.W. Neuropsychology of fear and loathing. Nat. Rev. Neurosci. 2, 352–363 (2001).
Dolan, R.J. & Morris, J.S. The functional anatomy of innate and acquired fear: perspectives from neuroimaging. in Cognitive Neuroscience of Emotion (eds. Lane, R.D. & Nadel, L.) 225–241 (Oxford Univ. Press, New York, 2000).
Funayama, E.S., Grillon, C., Davis, M. & Phelps, E.A. A double dissociation in the affective modulation of startle in humans: effects of unilateral temporal lobectomy. J. Cogn. Neurosci. 13, 721–729 (2001).
Morris, J.S., Ohman, A. & Dolan, R.J. Conscious and unconscious learning in the amygdala. Nature 393, 467–470 (1998).
Holstege, G. et al. Brain activation during human male ejaculation. J. Neurosci. 23, 9185–9193 (2003).
Talairach, J. & Tournoux, P. Co-planar Stereotaxic Atlas of the Human Brain (Thieme Medical, New York, 1988).
Duvernoy, H.M. The Human Brain: Surface, Three-dimensional Sectional Anatomy and MRI (Springer, New York, 1991).
Acknowledgements
This research was supported by the Center for Behavioral Neuroscience, a Science and Technology Center Program of the National Science Foundation, under agreement IBN-9876754.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Hamann, S., Herman, R., Nolan, C. et al. Men and women differ in amygdala response to visual sexual stimuli. Nat Neurosci 7, 411–416 (2004). https://doi.org/10.1038/nn1208
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1208
This article is cited by
-
The Perfect Paramour: Predicting Intention to Own a Sex Doll
Sexuality & Culture (2023)
-
Neuroelectric Correlates of Human Sexuality: A Review and Meta-Analysis
Archives of Sexual Behavior (2023)
-
Effect of Relationship Status on Response Times to Sexual and Romantic Stimuli Among Japanese Undergraduates in a Memory Task
Archives of Sexual Behavior (2022)