Abstract
Dopamine (DA) neurons in the ventral tegmental area (VTA) help mediate stress susceptibility and resilience. However, upstream mechanisms controlling these neurons remain unknown. Noradrenergic (NE) neurons in the locus coeruleus, implicated in the pathophysiology of depression, have direct connections within the VTA. Here we demonstrate that NE neurons regulate vulnerability to social defeat through inhibitory control of VTA DA neurons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Krishnan, V. et al. Cell 131, 391–404 (2007).
Cao, J.-L. et al. J. Neurosci. 30, 16453–16458 (2010).
Barik, J. et al. Science 339, 332–335 (2013).
Chaudhury, D. et al. Nature 493, 532–536 (2013).
Chandler, D.J., Lamperski, C.S. & Waterhouse, B.D. Brain Res. 1522, 38–58 (2013).
Guiard, B.P., El Mansari, M. & Blier, P. Mol. Pharmacol. 74, 1463–1475 (2008).
Golden, S.A., Covington, H.E. III, Berton, O. & Russo, S.J. Nat. Protoc. 6, 1183–1191 (2011).
George, S.A. et al. Eur. J. Neurosci. 37, 901–909 (2013).
Rusnák, M., Kvetnanský, R., Jeloková, J. & Palkovits, M. Brain Res. 899, 20–35 (2001).
Fan, Y., Chen, P., Li, Y. & Zhu, M.-Y. Synapse 67, 300–312 (2013).
Isingrini, E. et al. J. Psychiatry Neurosci. 41, 150028 (2015).
Russo, S.J. & Nestler, E.J. Nat. Rev. Neurosci. 14, 609–625 (2013).
Nestler, E.J. & Carlezon, W.A. Jr. Biol. Psychiatry 59, 1151–1159 (2006).
Goldstein, J.M., Knobloch, L.C. & Malick, J.B. Eur. J. Pharmacol. 91, 101–105 (1983).
Scheinin, H. & Virtanen, R. Life Sci. 39, 1439–1446 (1986).
Tye, K.M. et al. Nature 493, 537–541 (2013).
Grenhoff, J., North, R.A. & Johnson, S.W. Eur. J. Neurosci. 7, 1707–1713 (1995).
Lammel, S. et al. Nature 491, 212–217 (2012).
Friedman, A.K. et al. Science 344, 313–319 (2014).
Mongeau, R., de Montigny, C. & Blier, P. Neuropsychopharmacology 10, 41–51 (1994).
Bagot, R.C. et al. Nat. Commun. 6, 7062 (2015).
Paxinos George, F.K. The Mouse Brain in Stereotaxic Coordinates (Elsevier, 2008).
Freeman, A.S. & Bunney, B.S. Brain Res. 405, 46–55 (1987).
Grace, A.A. & Bunney, B.S. J. Neurosci. 4, 2877–2890 (1984).
Grace, A.A. & Bunney, B.S. J. Neurosci. 4, 2866–2876 (1984).
Amilhon, B. et al. Neuron 86, 1277–1289 (2015).
Rocchetti, J. et al. Biol. Psychiatry 77, 513–525 (2015).
Acknowledgements
We thank E. Vigneault and M.E. Desaulniers for care and maintenance of the mouse colonies. B.G. is supported by a Canadian Research Chair in the Neurobiology of Mental Disorders. E.I. is supported by a postdoctoral grant from the Fonds de Recherche du Québec - Santé. This work was supported by the Canada Research Chairs program, the Graham Boeckh Foundation for Schizophrenia Research and the Natural and Engineering Research Council of Canada (RGPIN 385732-2012) to B.G.
Author information
Authors and Affiliations
Contributions
The studies were conceived and designed by B.G. and E.I. Experiments were performed by E.I. and L.P. with contributions from Q.R. for in vivo optogenetic experiments; Q.R. and F.M. for in vivo electrophysiological recording; E.G., G.M. and J.R. for surgeries; A.G. and L.M. for neurochemical analysis; A.T. and N.M. for the histology; and B.A. and S.W. for patch-clamp recording. The paper was written by B.G., E.I. and L.P., and was edited by the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Chronic social defeat increases both neuronal excitability of the VTA DA system and DA levels in the NAc in susceptible but not in resilient mice.
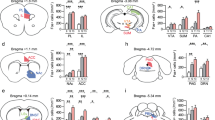
(a) Time (s) in the corner zone (F(2,73)= 24.8, p<0.001) was significantly increased during the second phase in the presence of CD1 mice (target) compared to the first phase with no target in susceptible mice (post-hoc ***p<0 .001). The time (s) spent in the corner zone with target was significantly increased in susceptible mice (n=29) compared with both control (n=30) and resilient mice (n=17) (post-hoc ***p<0.001). (b) Criterion of action potential waveform specific to a DA neuron (blue) with a long duration (>2.0ms) and a width form start to negative through > 1.1 ms compared to a non-DA neuron action potential (red). (c) Characteristics of a VTA-DA neuron burst activity with a burst starting with a latency between two action potentials <80ms and ended with a latency between two action potentials >160ms. (d) Example of recording track into the VTA. (e) Representative trace of pharmacological characterization of a VTA-DA neuron. (f) Sample traces for in vivo recording of VTA-DA neurons in control, susceptible and resilient mice. (g) The firing rate (Hz ; F(2,98)= 4.95, p=0.009 ; post-hoc *p=0.042, **p=0.0025 ; right), the number of bursts per minute (F(2,98)= 7.04, p=0.0014 ; post-hoc **p=0.0033, ***p=0.0007 ; middle) and the percentage of spikes within burst (%SWB ; F(2,98)= 3.68, p=0.029 ; post-hoc *p<0.05 ; right) was significantly increased in susceptible mice (n=32) to social defeat compared with both control mice (n=34) and resilient mice (n=35). (h) The level of DA (µg/g) in the NAc (H(2, 20)=3.65, p=0.44 ; right), but not in the PFC (F(2, 20)=0.89, p=0.42 ; left), was significantly decreased in resilient mice (n=8) compared with susceptible mice (n=7) (control mice n=9; post-hoc *p=0.014).
Supplementary Figure 2 Chronic social defeat decreases c-fos expression in the LC of susceptible mice.
(a) Illustration of c-fos DAB immunostained positive cells in the LC (left; scale bars: left 100 µm, right 50 µm). This staining has been reproduced on 12 mice. Quantification of c-fos positive cells in the LC (relative % to control) showed a significant decrease in susceptible mice (n=4) compared with control mice (n=3) (resilient mice n=4; F(2,7)=7.72, p=0.017; post-hoc **p=0.0057, right). (b) Illustration of retrograde tracer bilaterally injected into the VTA. (c) Representative photographs of the TH/c-fos/Beads triple labelling in the LC of a control mouse (left, scale bars: 50 µm) with high magnification (right, scale bars: 10 µm) of a c-fos/TH/Beads cell (upper, yellow arrow), a c-fos/Beads/NonTH cell (middle, red arrow) and a c-fos/TH/NonBeads cell (lower, green arrow). This staining has been reproduced on 24 mice. (d) Number of c-fos positive cells per µm2 in the LC, co-expressing or not TH and/or the beads retrograde markers injected into the VTA in control (n=9), susceptible (n=7) and resilient (n=8) mice to chronic social defeat (F(2,20)=5.8, p=0.015 ; post-hoc *p=0.005 in comparison with control and p=0.043 in comparison with resilient mice).
Supplementary Figure 3 Validation of the conditional VMAT2DBHcre knockout mice with brain-specific NE depletion.
(a) Picture of the cre-recombinase expression in the LC of transgenic DBHcre mice crossed with TdTomato reporter mice. (b) The autoradiographic distribution of VMAT2 mRNA measured by in situ hybridization shed light on Cre-excision effectiveness of VMAT2 specifically in the LC of the KO mice. The absence of VMAT2 mRNA in the LC has been reproduced on 3 KO mice. (c) The whole brain levels of monoamines (µg/g) measured by HPLC indicated specific NE level alterations in KO mice (n=3 per group; t=22.4, ***p<0.001). DA level (t=0.16, p=0.88) and 5HT level (t=-0.33, p=0.76) were similar between WT and KO. (d) KO mice were born from double heterozygote crosses with the expected Mendelian ratio of 3/16 (18.75%). (e) Spontaneous locomotion in a new environment measured at 5-minute intervals for 3 hours was similar between KO and WT mice (n=8 per group; F(35,490)=0.54, p=0.99, ns). (f) Motor coordination observed in the rotarod for 3 consecutive sessions was unaltered in KO mice compared with WT (n=8 per group; F(2,28)=0.4, p=0.67, ns).
Supplementary Figure 4 Detailed results of the social interaction test in the VMAT2DBHcre KO mice.
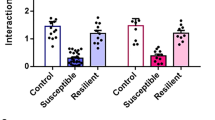
(a) Ten days of social defeat induce social avoidance in WT and KO mice, however no resilient group was observed in KO mice. Whereas the social interaction time (s) in absence of target was similar between all WT and KO groups, the social interaction time (s) with target was significantly decreased in susceptible WT (n=16) and KO (n=19) mice compared with their respective control (n=24 per control group; F(4,84)=27.5, p<0.001 ; post-hoc ***p<0.001). (b) The social interaction ratio (Target/NoTarget) was significantly decreased in susceptible WT and KO mice compared with their respective control (F(4,83)=12.58, p<0.001; post-hoc ***p<0.001). (c) Whereas the time spent in the corner zone (s) in absence of target was similar between all WT and KO groups, the time spent in the corner zone (s) in presence of the target was significantly increased in susceptible WT and KO mice compared with their respective control (F(4,84)=3.2, p=0.017; post-hoc *p=0.011, **p=0.007).
Supplementary Figure 5 Detailed results of the social interaction test in control and susceptible mice treated with either acute or chronic idazoxan.
(a) In susceptible mice, the time spent (s) in the interaction zone with target was significantly lower than the time spent (s) with no target in both the NaCl (n=10) and the idazoxan-treated (n=8) groups (Target effect: post-defeat F(1,16)=24.44, p=0.00015, post-hoc ***p<0.00019 and acute treatment F(1,16)=5.9, p=0.027, post-hoc *p=0.039). However, no differences between groups were observed during the two sessions. (b) After 1 week of treatment, the correlation between the interaction score (Target/NoTarget) and the time spent in the interaction zone (s) with the target showed that 10% (1/10) of the NaCl-treated mice spent more than 60s interacting with the target compared with 75% (6/8) of the idazoxan-treated mice. (c) In control animals, either acute or chronic idazoxan (2 mg/kg, i.p.) treatment had no effect on the time (s) spent in the interaction zone with target (F(2,42)=0.17, p=0.85, left). The time spent in the interaction zone (s) with target was either increased (post-defeat, ***p<0.001) or similar (Acute and 1 week) than the time spent in the interaction zone (s) with no target in both the control NaCl (n=11) and idazoxan-treated (n=12) groups. However, no differences between control groups were observed during the two sessions and the three timepoints.
Supplementary Figure 6 Chronic reboxetine treatment reverses a susceptible phenotype to social defeat and acute effect of idazoxan on neuronal excitability of the VTA DA system.
(a) After 10 days of social defeat, 1 week treatment with reboxetine (20 mg/kg, i.p.) in susceptible mice counteracted this phenotype to induce a resilient-like phenotype. After 1 week of treatment, the interaction time (s) with the CD1 of the susceptible reboxetine-treated mice (n=8) was significantly increased compared to susceptible NaCl-treated mice (n=15) (F(1,41)=3.95, p=0.05 ; post-hoc *p<0.05, **p=0.0067) and was similar to control groups (NaCl n=15, Reboxetine n=7). (b) The DA neurons firing rate (Hz; right) was not modified after acute idazoxan injection (2 mg/kg, i.p.) compared with baseline (n=7; F(7,57)=1.51, p=0.18, ns). (c) The number of bursts per minute was not modified after acute idazoxan injection (2 mg/kg, i.p.) compared with baseline (F(7,57)=1.13, p=0.36, ns). (d) The percentage of spikes within burst (%SWB) was significantly increased between 25 and 35 minutes after idazoxan injection (2 mg/kg, i.p.) compared to baseline (F(7,57)=4.59, p<0.001, post-hoc *p<0.05 compared with baseline).
Supplementary Figure 7 In vitro and in vivo experimental validation for optogenetic stimulation of the LC NE fibers in the VTA.
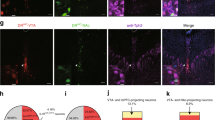
(a) Photomicrograph of the LC of DBHcre-TdTomato mice transduced with the virus AAV1-ChR2-eYFP showing co-localization of DBH positive neurons (Tomato red staining) and ChR2 expressing cells (eYFP green staining; scale bars: upper 50 µm, lower 20 µm). 89.9% of DBH positive cells co-expressed the ChR2-eYFP in the LC and DBH-Tdtomato cells could be detected in 97.2% of the ChR2-eYFP expressing cells (n=3 mice). (b) Photomicrograph of the VTA of DBHcre-TdTomato mice transduced with the virus AAV1-ChR2-eYFP showing co-localization of DBH positive fibers (Tomato red staining) and ChR2 expressing fibers (eYFP green staining). The triple labelling has been reproduced on 13 mice. TH immunostaining (blue) showed DA neurons contacting the NE fibers from the LC expressing the ChR2. Scale bars: left 100 µm, right 10 µm. (c) Example electrophysiological characterization of a TdTomato- and eYFP-positive noradrenergic neuron in the LC. NE neurons fired on average at 15.1 ± 1.1 Hz in response to a 180 pA depolarizing step. A large majority of NE neurons were spontaneously active (n = 3 mice, 10 out of 11 neurons) and fired on average at 3.1 ± 0.5 Hz. (d) Example of photocurrents recorded in voltage-clamp (holding = -70 mV) during a 500 ms continuous blue-light stimulation in a LC-NE ChR2-positive neuron. (e) Steady-state photocurrent size quantification. Photocurrents were on average 271.0 ± 89.2 pA (n = 3 mice, n = 12 neurons). (f) Example traces of blue-light stimulations of a LC-NE ChR2-positive neuron. In these examples, square pulses of 5 ms duration were delivered at 5 and 20 Hz. (g) Action potential fidelity when stimulating LC-NE ChR2-positive neurons with 5 ms blue-light pulses at frequencies 1-50 Hz. As expected considering NE neuron electrophysiological properties, reliable firing (> 75%) was observed for stimulation frequencies between 1 and 10 Hz (n = 3 mice, n = 8 neurons). (h) Photomicrograph of activated LC-NE neurons (c-fos, blue) of DBHcre-TdTomato (red) mice transduced with the virus AAV1-ChR2-eYFP or AAV1- eYFP (green) which received blue-light optical stimulation (10-ms at 10 Hz blue light pulses over 500 ms every 20 seconds) in the LC for 20 minutes. This experiment has been reproduced successfully on 6 mice per condition. Scale bars: 50 µm.
Supplementary Figure 8 Detailed results of the social interaction test in control and susceptible mice with either acute or chronic optogenetic stimulation of the LC NE fibers in the VTA.
(a) In susceptible mice, the time spent in the interaction zone with target was significantly lower (post-defeat F(1,10)=21.7, p<0.001; post-hoc ***p=0.0009) or similar (acute F(1,10)=2.85, p=0.012) than the time spent with no target in both the YFP (n=6) and the ChR2-stimulated (n=6) groups. However, no differences between groups were observed during the two sessions. (b) After 1 week of stimulation, the correlation between the interaction score and the time spent in the interaction zone with the target showed that none (0/6) of the YFP-stimulated mice spent more than 60s interacting with the target compared with 66.6% (4/6) in the ChR2-stimulated mice. (c) In control animals, either acute or chronic stimulation of the LC-NE fibers in the VTA had no effect on the time spent in the interaction zone with target (F(2,28)=0.26, p=0.78, left). The time spent in the interaction zone with target was either increased (post-defeat, F(1,14)=15.98, p=0.0013; post-hoc **p<0.0013) or similar (Acute F(1,14)=0.002, p=0.97 and 1 week F(1,14)=0.06, p=0.0.81) than the time spent in the interaction zone with no target in both the control YFP (n=9) and ChR2-stimulated (n=7) groups. However, no differences between control groups were observed during the two sessions and the three timepoints.
Supplementary information
Supplementary Text and Figures
Supplementary figures 1–8 (PDF 1734 kb)
Source data
Rights and permissions
About this article
Cite this article
Isingrini, E., Perret, L., Rainer, Q. et al. Resilience to chronic stress is mediated by noradrenergic regulation of dopamine neurons. Nat Neurosci 19, 560–563 (2016). https://doi.org/10.1038/nn.4245
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4245
This article is cited by
-
Central regulation of stress-evoked peripheral immune responses
Nature Reviews Neuroscience (2023)
-
The laterodorsal tegmentum-ventral tegmental area circuit controls depression-like behaviors by activating ErbB4 in DA neurons
Molecular Psychiatry (2023)
-
Effects of the CALM intervention on resilience in Chinese patients with early breast cancer: a randomized trial
Journal of Cancer Research and Clinical Oncology (2023)
-
Cav3.1-driven bursting firing in ventromedial hypothalamic neurons exerts dual control of anxiety-like behavior and energy expenditure
Molecular Psychiatry (2022)
-
A novel red fluorescence dopamine biosensor selectively detects dopamine in the presence of norepinephrine in vitro
Molecular Brain (2021)