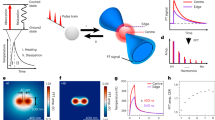
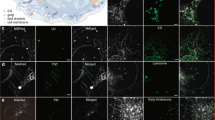
Abstract
Lipid bodies have an important role in energy storage and lipid regulation. Here we show that lipid bodies are a major source of contrast in third-harmonic generation (THG) microscopy of cells and tissues. In hepatocytes, micrometer-sized lipid bodies produce a THG signal 1–2 orders of magnitude larger than other structures, which allows one to image them with high specificity. THG microscopy with ∼1,200 nm excitation can be used to follow the distribution of lipid bodies in a variety of unstained samples including insect embryos, plant seeds and intact mammalian tissue (liver, lung). We found that epi-THG imaging is possible in weakly absorbing tissues because bulk scattering redirects a substantial fraction of the forward-generated harmonic light toward the objective. Finally, we show that the combination of THG microscopy with two-photon and second-harmonic imaging provides a new tool for exploring the interactions between lipid bodies, extracellular matrix and fluorescent compounds (vitamin A, NADH and others) in tissues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Murphy, D.J. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog. Lipid Res. 40, 325–438 (2001).
Martin, S. & Parton, R.G. Caveolin, cholesterol, and lipid bodies. Semin. Cell Dev. Biol. 16, 163–174 (2005).
Murphy, D.J. & Vance, J. Mechanisms of lipid-body formation. Trends Biochem. Sci. 24, 109–115 (1999).
Imanishi, Y., Gerke, V. & Palczewski, K. Retinosomes: new insights into intracellular managing of hydrophobic substances in lipid bodies. J. Cell Biol. 166, 447–453 (2004).
Heid, H.W. & Keenan, T.W. Intracellular origin and secretion of milk fat globules. Eur. J. Cell Biol. 84, 245–258 (2005).
Greenspan, P., Mayer, E.P. & Fowler, S.D. Nile red: a selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 100, 965–973 (1985).
Zipfel, W.R., Williams, R.M. & Webb, W.W. Nonlinear magic: multiphoton microscopy in the biosciences. Nat. Biotechnol. 21, 1369–1377 (2003).
Zipfel, W.R. et al. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second-harmonic generation. Proc. Natl. Acad. Sci. USA 100, 7075–7080 (2003).
Barad, Y., Eisenberg, H., Horowitz, M. & Silberberg, Y. Nonlinear scanning laser microscopy by third harmonic generation. Appl. Phys. Lett. 70, 922–924 (1997).
Squier, J.A., Müller, M., Brakenhoff, G.J. & Wilson, K.R. Third harmonic generation microscopy. Opt. Express 3, 315–324 (1998).
Oron, D. et al. Depth-resolved structural imaging by third-harmonic generation microscopy. J. Struct. Biol. 147, 3–11 (2004).
Boyd, R.W. Nonlinear optics 2nd edn. (Academic Press, San Diego, 2003).
Cheng, J.-X. & Xie, X.S. Green's function formulation for third harmonic generation microscopy. J. Opt. Soc. Am. B 19, 1604–1610 (2002).
Débarre, D., Supatto, W. & Beaurepaire, E. Structure sensitivity in third-harmonic generation microscopy. Opt. Lett. 30, 2134–2136 (2005).
Kajzar, F. & Messier, J. Original technique for third-harmonic-generation measurements in liquids. Rev. Sci. Instr. 58, 2081–2085 (1987).
Delahunty, T.J. & Rubinstein, D. Accumulation and release of triglycerides by rat liver following partial hepatectomy. J. Lipid Res. 11, 536–543 (1970).
Shteyer, E., Liao, Y., Muglia, L.J., Hruz, P.W. & Rudnick, D.A. Disruption of hepatic adipogenesis is associated with impaired liver regeneration in mice. Hepatology 40, 1322–1332 (2004).
Welte, M.A., Gross, S.P., Postner, M., Block, S.M. & Wieschaus, E.F. Developmental regulation of vesicle transport in Drosophila embryos: forces and kinetics. Cell 92, 547–557 (1998).
Débarre, D. et al. Velocimetric third-harmonic generation microscopy: micrometer-scale quantification of morphogenetic movements in unstained embryos. Opt. Lett. 29, 2881–2883 (2004).
Sun, C.-K. et al. Higher harmonic generation microscopy for developmental biology. J. Struct. Biol. 147, 19–30 (2004).
Supatto, W. et al. In vivo modulation of morphogenetic movements in Drosophila embryos with femtosecond laser pulses. Proc. Natl. Acad. Sci. USA 102, 1047–1052 (2005).
Cox, G., Moreno, N. & Feijo, J. Second-harmonic imaging of plant polysaccharides. J. Biomed. Opt. 10, 024013 (2005).
Beaurepaire, E. & Mertz, J. Epifluorescence collection in two-photon microscopy. Appl. Opt. 41, 5376–5382 (2002).
Oheim, M., Beaurepaire, E., Chaigneau, E., Mertz, J. & Charpak, S. Two-photon microscopy in brain tissue: parameters influencing the imaging depth. J. Neurosci. Methods 111, 29–37 (2001).
Dietl, P. & Haller, T. Exocytosis of lung surfactant: from the secretory vesicle to the air-liquid interface. Annu. Rev. Physiol. 67, 595–621 (2005).
Dirami, G. et al. Lung retinol storing cells synthesize and secrete retinoic acid, an inducer of alveolus formation. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L249–L256 (2004).
Huang, S., Heikal, A.A. & Webb, W.W. Two-photon fluorescence spectroscopy and microscopy of NAD(P)H and flavoprotein. Biophys. J. 82, 2811–2825 (2002).
Patterson, G.H., Knobel, S.M., Arkhammar, P., Thastrup, O. & Piston, D.W. Separation of the glucose-stimulated cytoplasmic and mitochondrial NAD(P)H reponses in pancreatic islet beta cells. Proc. Natl. Acad. Sci. USA 97, 5203–5207 (2000).
Nan, X., Cheng, J.-X. & Xie, X.S. Vibrational imaging of lipid droplets in live fibroblast cells with coherent anti-Stokes Raman scattering microscopy. J. Lipid Res. 44, 2202–2208 (2003).
Niemz, M.H. Laser-Tissue Interactions: fundamentals and Applications (Springer, Berlin, 2004).
Theer, P., Hasan, M.T. & Denk, W. Two-photon imaging to a depth of 1000 μm in living brains by use of a TiAl2O3 regenerative amplifier. Opt. Lett. 28, 1022–1024 (2003).
Beaurepaire, E., Oheim, M. & Mertz, J. Ultra-deep two-photon excitation in turbid media. Opt. Commun. 188, 25–29 (2001).
Day, C.P. & James, O.F.W. Hepatic steatosis: Innocent bystander or guilty party? Hepatology 27, 1463–1466 (1998).
Bosshard, C., Gubler, U., Kaatz, P., Mazerant, W. & Meir, U. Non-phase-matched optical third-harmonic generation in noncentrosymmetric media: cascaded second-order contributions for the calibration of third-order nonlinearities. Phys. Rev. B 61, 10688–10701 (2000).
Wohlfarth, C. & Wohlfarth, B. Landolt-Börnstein Numerical data and functional relationships in science and technology (New Series III/38A et B) (Springer-Verlag, Berlin, 1996).
Nikogosyan, N.D. Properties of Optical and Laser-related materials: a handbook (Wiley, Chichester, 1997).
Acknowledgements
We thank M. Welte (Brandeis University, Waltham, Massachusetts, USA) for advice on embryo staining, G. Ephritikhine (Institut des Sciences du Végétal, Gif-sur-Yvette, France) for help with the plant experiments, J.-M. Sintes and X. Solinas for assistance in microscope design, S. Boucherie for assistance in hepatocytes preparation, and J.-L. Martin and J. Ogilvie for critical comments. This work was supported by the Délégation Générale pour l'Armement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
THG imaging of isolated hepatocytes in different physiological states. (PDF 46 kb)
Supplementary Figure 2
THG-2PEF imaging of plant seed tissue labeled with Nile Red. (PDF 170 kb)
Supplementary Figure 3
Fluorescence emission spectrum of lipid bodies in lung tissue. (PDF 13 kb)
Supplementary Video 1
Simultaneous THG(purple)-2PEF(red) 3D imaging of a hepatocyte stained with Nile Red, a hydrophobic fluorescent dye that accumulates in lipid bodies. (MOV 1866 kb)
Supplementary Video 2
THG image sequence showing the trafficking dynamics of lipid bodies in a live D. melanogaster embryo during cellularization. (MOV 2149 kb)
Supplementary Video 3
Individual lipid body tracked using THG microscopy in a developing D. melanogaster embryo. (MOV 1393 kb)
Supplementary Data
Cell viability after THG imaging. (PDF 297 kb)
Rights and permissions
About this article
Cite this article
Débarre, D., Supatto, W., Pena, AM. et al. Imaging lipid bodies in cells and tissues using third-harmonic generation microscopy. Nat Methods 3, 47–53 (2006). https://doi.org/10.1038/nmeth813
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth813
This article is cited by
-
The Cousa objective: a long-working distance air objective for multiphoton imaging in vivo
Nature Methods (2024)
-
Multiscale spectroscopic analysis of lipids in dimorphic and oleaginous Mucor circinelloides accommodate sustainable targeted lipid production
Fungal Biology and Biotechnology (2023)
-
Multiphoton microscopy is a nondestructive label-free approach to investigate the 3D structure of gas cell walls in bread dough
Scientific Reports (2023)
-
Third harmonic imaging contrast from tubular structures in the presence of index discontinuity
Scientific Reports (2023)
-
Label-free imaging of red blood cells and oxygenation with color third-order sum-frequency generation microscopy
Light: Science & Applications (2023)