Abstract
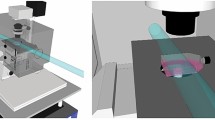
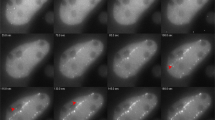
Understanding how cells maintain genome integrity when challenged with DNA double-strand breaks (DSBs) is of major importance, particularly since the discovery of multiple links of DSBs with genome instability and cancer-predisposition disorders1,2. Ionizing radiation is the agent of choice to produce DSBs in cells3; however, targeting DSBs and monitoring changes in their position over time can be difficult. Here we describe a procedure for induction of easily recognizable linear arrays of DSBs in nuclei of adherent eukaryotic cells by exposing the cells to α particles from a small Americium source (Box 1). Each α particle traversing the cell nucleus induces a linear array of DSBs, typically 10–20 DSBs per 10 μm track length4. Because α particles cannot penetrate cell-culture plastic or coverslips, it is necessary to irradiate cells through a Mylar membrane. We describe setup and irradiation procedures for two types of experiments: immunodetection of DSB response proteins in fixed cells grown in Mylar-bottom culture dishes (Option A) and detection of fluorescently labeled DSB-response proteins in living cells irradiated through a Mylar membrane placed on top of the cells (Option B). Using immunodetection, recruitment of repair proteins to individual DSB sites as early as 30 s after irradiation can be detected. Furthermore, combined with fluorescence live-cell microscopy of fluorescently tagged DSB-response proteins, this technique allows spatiotemporal analysis of the DSB repair response in living cells. Although the procedures might seem a bit intimidating, in our experience, once the source and the setup are ready, it is easy to obtain results. Because the live-cell procedure requires more hands-on experience, we recommend starting with the fixed-cell application.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Shiloh, Y. ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer 3, 155–168 (2003).
Gudmundsdottir, K. & Ashworth, A. The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene 43, 5864–5874 (2006).
Essers, J., Houtsmuller, A.B. & Kanaar, R. Analysis of DNA recombination and repair proteins in living cells by photobleaching microscopy. Methods Enzymol. 408, 463–485 (2006).
Aten, J.A. et al. Dynamics of DNA double-strand breaks revealed by clustering of damaged chromosome domains. Science 303, 92–95 (2004).
Stap, J., Van Marle, J. & Van Veen, H.A. Coating of coverslips with glow-discharged carbon promotes cell attachment and spreading probably due to carboxylic groups. Cytometry 39, 295–299 (2000).
Van Veelen, L.R., Kanaar, R. & van Gent, D.C. DNA repair and malignant hematopoiesis. in Textbook of Malignant Hematology 2nd edn. (L. Degos, D.C. Linch & B. Löwenberg, eds.) 155–164 (Taylor & Francis Group, London, 2005).
Dinant, C. et al. Activation of multiple DNA repair pathways by sub nuclear damage induction methods. J. Cell Sci. 120, 2731–2740 (2007).
Limoli, C.L. & Ward, J.F. A new method for introducing double-strand breaks into cellular DNA. Radiat. Res. 134, 160–169 (1993).
Durand, R.E. & Olive, P.L. Cytotoxicity, mutagenicity and DNA damage by Hoechst 33342. J. Histochem. Cytochem. 30, 11–16 (1982).
Bradshaw, P.S., Stavropoulos, D.J. & Meyn, M.S. Human telomeric protein TRF2 associates with genomic double-strand breaks as an early response to DNA damage. Nat. Genet. 37, 193–197 (2005).
Williams, E.S. et al. DNA double strand breaks are not sufficient to initiate recruitment of TRF2. Nat. Genet. 39, 696–698 (2007).
Fulford, J., Nikjoo, H., Goodhead, D.T. & O'Neill, P. Yields of SSB and DSB induced in DNA by ALK ultrasoft X-rays and α particles: comparison of experimental and simulated yields. Int. J. Radiat. Biol. 77, 1053–1066 (2001).
Barendsen, G.W. The relationships between RBE and LET for different types of lethal damage in mammalian cells: biophysical and molecular mechanisms. Radiat. Res. 139, 257–270 (1994).
Barendsen, G.W. Dose-survival curves of human cells in tissue culture irradiated with alpha-, beta-, 20-KV X- and 200-KV X-radiation. Nature 193, 1153–1155 (1962).
Hoebe, R.A. et al. Controlled light-exposure microscopy reduces photobleaching and photoxicity in fluorescence live-cell imaging. Nat. Biotechnol. 25, 249–253 (2007).
Bekker-Jensen, S. et al. Spatial organization of the mammalian genome surveillance machinery in response to DNA strand breaks. J. Cell Biol. 173, 195–206 (2006).
Acknowledgements
We thank R.A. Hoebe, R.G. Tigchelaar, J. Pos, M. Rijpkema, H.W. van Ijzendoorn and J.J. Pijnenborg for technical assistance. This work was funded in part by grants from the Dutch Cancer Society (J.S., P.M.K., J.E., R.K. and J.A.A.), the Netherlands Genomic Initiative/Netherlands Organization for Scientific Research and the European Commission (IP 512113).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3, Supplementary Methods (PDF 840 kb)
Supplementary Movie
Supplementary Video 1 (MOV 297 kb)
Rights and permissions
About this article
Cite this article
Stap, J., Krawczyk, P., Van Oven, C. et al. Induction of linear tracks of DNA double-strand breaks by α-particle irradiation of cells. Nat Methods 5, 261–266 (2008). https://doi.org/10.1038/nmeth.f.206
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.f.206
This article is cited by
-
Characterization of γ-H2AX foci formation under alpha particle and X-ray exposures for dose estimation
Scientific Reports (2022)
-
A simple microscopy setup for visualizing cellular responses to DNA damage at particle accelerator facilities
Scientific Reports (2021)
-
DNA DSB Repair Dynamics following Irradiation with Laser-Driven Protons at Ultra-High Dose Rates
Scientific Reports (2019)
-
DNA damage in leukocytes after internal ex-vivo irradiation of blood with the α-emitter Ra-223
Scientific Reports (2018)
-
Single α-particle irradiation permits real-time visualization of RNF8 accumulation at DNA damaged sites
Scientific Reports (2017)