Abstract
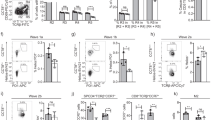
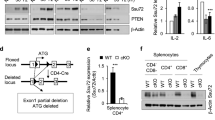
Engagement of antigen receptors triggers the proliferation and functional activation of lymphocytes. Here we report that T cell homeostasis and antigen-induced responses require the COP9 signalosome (CSN), a regulator of the ubiquitin-proteasome system. Conditional deletion of the CSN subunit Csn8 in peripheral T lymphocytes disrupted formation of the CSN complex, reduced T cell survival and proliferation in vivo and impaired antigen-induced production of interleukin 2. Moreover, Csn8-deficient T cells showed defective entry into the cell cycle from the G0 quiescent state. This phenotype was associated with a lack of signal-induced expression of cell cycle–related genes, including G1 cyclins and cyclin-dependent kinases, and with excessive induction of p21Cip1. Our data define a CSN-dependent pathway of transcriptional control that is essential for antigen-induced initiation of T cell proliferation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Dong, C., Davis, R.J. & Flavell, R.A. MAP kinases in immune response. Annu. Rev. Immunol. 20, 55–72 (2002).
Ruland, J. & Mak, T.W. From antigen to activation: specific signal transduction pathways linking antigen receptors to NFκB. Semin. Immunol. 15, 177–183 (2003).
Rowell, E.A. & Wells, A.D. The role of cyclin-dependent kinases in T-cell development, proliferation and function. Crit. Rev. Immunol. 26, 189–212 (2006).
Sage, J., Miller, A.L., Perez-Mancera, P.A., Wysocki, J.M. & Jacks, T. Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature 424, 223–228 (2003).
Cobrinik, D. Pocket proteins and cell cycle control. Oncogene 24, 2796–2809 (2005).
Ren, S. & Rollins, B.J. Cyclin C/cdk3 promotes Rb-dependent G0 exit. Cell 117, 239–251 (2004).
Boylan, J.F., Sharp, D.M., Leffet, L., Bowers, A. & Pan, W. Analysis of site-specific phosphorylation of the retinoblastoma protein during cell cycle progression. Exp. Cell Res. 248, 110–114 (1999).
Sherr, C.J. & Roberts, J.M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13, 1501–1512 (1999).
Lea, N.C. et al. Commitment point during G0 → G1 that controls entry into the cell cycle. Mol. Cell. Biol. 23, 2351–2361 (2003).
Grumont, R. et al. The mitogen-induced increase in T cell size involves PKC and NFAT activation of Rel/NF-κB-dependent c-Myc expression. Immunity 21, 19–30 (2004).
Liu, Y.C. Ubiquitin ligases and the immune response. Annu. Rev. Immunol. 22, 81–127 (2004).
Wei, N. & Deng, X.W. The COP9 signalosome. Annu. Rev. Cell Dev. Biol. 19, 261–286 (2003).
Bech-Otschir, D., Seeger, M. & Dubiel, W. The COP9 signalosome: at the interface between signal transduction and ubiquitin-dependent proteolysis. J. Cell Sci. 115, 467–473 (2002).
Zhou, C. et al. Fission yeast COP9/Signalosome suppresses cullin activity through recruitment of the deubiquitylating enzyme Ubp12p. Mol. Cell 11, 927–938 (2003).
Lyapina, S. et al. Promotion of NEDD-CUL1 conjugate cleavage by COP9 signalosome. Science 292, 1382–1385 (2001).
Schwechheimer, C. et al. Interactions of the COP9 signalosome with the E3 ubiquitin ligase SCFTIRI in mediating auxin response. Science 292, 1379–1382 (2001).
Pan, Z.Q., Kentsis, A., Dias, D.C., Yamoah, K. & Wu, K. Nedd8 on cullin: building an expressway to protein destruction. Oncogene 23, 1985–1997 (2004).
Cope, G.A. et al. Role of predicted metalloprotease motif of Jab1/Csn5 in cleavage of Nedd8 from Cul1. Science 298, 608–611 (2002).
Mundt, K.E., Liu, C. & Carr, A.M. Deletion mutants in COP9/signalosome subunits in fission yeast schizosaccharomyces pombe display distinct phenotypes. Mol. Biol. Cell 13, 493–502 (2002).
Liu, C. et al. Cop9/signalosome subunits and Pcu4 regulate ribonucleotide reductase by both checkpoint-dependent and -independent mechanisms. Genes Dev. 17, 1130–1140 (2003).
Wang, X. et al. CSN1 N-terminal-dependent activity is required for Arabidopsis development but not for Rub1/Nedd8 deconjugation of cullins: a structure-function study of CSN1 subunit of COP9 signalosome. Mol. Biol. Cell 13, 646–655 (2002).
Rosel, D. & Kimmel, A.R. The COP9 signalosome regulates cell proliferation of Dictyostelium discoideum. Eur. J. Cell Biol. 85, 1023–1034 (2006).
Wei, N. & Deng, X.W. COP9: a new genetic locus involved in light-regulated development and gene expression in Arabidopsis. Plant Cell 4, 1507–1518 (1992).
Lykke-Andersen, K. et al. Disruption of the COP9 signalosome Csn2 subunit in mice causes deficient cell proliferation, accumulation of p53 and cyclin E and early embryonic death. Mol. Cell. Biol. 23, 6790–6797 (2003).
Yan, J. et al. COP9 signalosome subunit 3 is essential for maintenance of cell proliferation in the mouse embryonic epiblast. Mol. Cell. Biol. 23, 6798–6808 (2003).
Tomoda, K., Yoneda-Kato, N., Fukumoto, A., Yamanaka, S. & Kato, J.Y. Multiple functions of Jab1 are required for early embryonic development and growth potential in mice. J. Biol. Chem. 279, 43013–43018 (2004).
Lee, P.P. et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 15, 763–774 (2001).
Surh, C.D. & Sprent, J. Homeostatic T cell proliferation: how far can T cells be activated to self ligands? J. Exp. Med. 192, F9–F14 (2000).
Jameson, S.C. Maintaining the norm: T-cell homeostasis. Nat. Rev. Immunol. 2, 547–556 (2002).
Tough, D.F. & Sprent, J. Turnover of naive and memory-phenotype T cells. J. Exp. Med. 179, 1127–1135 (1994).
Fry, T.J. & Mackall, C.L. The many faces of IL7: from lymphopoiesis to peripheral T cell maintenance. J. Immunol. 174, 6571–6576 (2005).
Gudmundsdottir, H., Wells, A.D. & Turka, L.A. Dynamics and requirements of T cell clonal expansion in vivo at the single-cell level: effector function is linked to proliferative capacity. J. Immunol. 162, 5212–5223 (1999).
Bjorklund, M. et al. Identification of pathways regulating cell size and cell-cycle progression by RNAi. Nature 439, 1009–1013 (2006).
Cope, G.A. & Deshaies, R.J. Targeted silencing of Jab1/Csn5 in human cells downregulates SCF activity through reduction of F-box protein levels. BMC Biochem. 9, 1 (2006).
Denti, S., Fernandez Sanchez, M.E., Rogge, L. & Bianchi, E. The COP9 signalsome regulates Skp2 levels and proliferation of human cells. J. Biol. Chem. 281, 32188–32196 (2006).
Valerio Dorrello, N. et al. SCFßTRCP- and S6K1-mediated degradation of PDCD4 promotes protein translation and cell growth. Science 314, 467–471 (2006).
Kim, S.Y., Herbst, A., Tworkowski, K.A., Salghetti, S.E. & Tansey, W.P. Skp2 regulates Myc protein stability and activity. Mol. Cell 11, 1177–1188 (2003).
Von der Lehr, N. et al. The F-box protein Skp2 participates in c-Myc proteosomal degradation and acts as a cofactor for c-Myc-regulated transcription. Mol. Cell 11, 1189–1200 (2003).
Welcker, M. et al. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc. Natl. Acad. Sci. USA 101, 9085–9090 (2004).
Yada, M. et al. Phosphoryltion-dependent degradation of c-Myc is mediated by F-box protein Fbw7. EMBO J. 23, 2116–2125 (2004).
Bouchard, C. et al. Regulation of cyclin D2 gene expression by the Myc/Max/Mad network: Myc-dependent TRRAP recruitment and histone acetylation at the cyclin D2 promoter. Genes Dev. 15, 2042–2047 (2001).
Wei, N. & Deng, X.W. Characterization and purification of the mammalian COP9 complex, a conserved nuclear regulator initially identified as a repressor of photomorphogenesis in higher plants. Photochem. Photobiol. 68, 237–241 (1998).
Yoneda-Kato, N., Tomoda, K., Umehara, M., Arata, Y. & Kato, J.Y. Myeloid leukemia factor 1 regulates p53 by suppressing COP1 via COP9 signalosome subunit 3. EMBO J. 24, 1739–1749 (2005).
Yang, X. et al. The COP9 signalosome inhibits p27(kip1) degradation and impedes G1-S phase progression via deneddylation of SCF Cul1. Curr. Biol. 12, 667–672 (2002).
Inoue, Y., Kitagawa, M. & Taya, Y. Phosphorylation of pRB at Ser612 by Chk1/2 leads to a complex between pRB and E2F-1 after DNA damage. EMBO J. 26, 2083–2093 (2007).
Tomoda, K., Kubota, Y. & Kato, J. Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature 398, 160–165 (1999).
Tomoda, K. et al. The Jab1/COP9 signalosome subcomplex is a downstream mediator of Bcr-Abl kinase activity and facilitates cell-cycle progression. Blood 105, 775–783 (2005).
Fukumoto, A., Tomoda, K., Kubota, M., Kato, J.Y. & Yoneda-Kato, N. Small Jab1-containing subcomplex is regulated in an anchorage- and cell cycle-dependent manner, which is abrogated by ras transformation. FEBS Lett. 579, 1047–1054 (2005).
Oron, E. et al. COP9 signalosome subunits 4 and 5 regulate multiple pleiotropic pathways in Drosophila melanogaster. Development 129, 4399–4409 (2002).
Ullah, Z., Buckley, M.S., Arnosti, D.N. & Henry, R.W. Retinoblastoma protein regulation by the COP9 signalosome. Mol. Biol. Cell 18, 1179–1186 (2007).
Collins, G.A. & Tansey, W.P. The proteasome: a utility tool for transcription? Curr. Opin. Genet. Dev. 16, 197–202 (2006).
Acknowledgements
We thank R. Reed (Harvard Medical School) for anti-SF3a; H. Zhang (Yale Medical School) for anti–cyclin A; N. Colburn (National Institutes of Health) for anti-Pdcd4; R. Pardi for communicating unpublished data; K. Lykke-Andersen for contributions to the design and initial construction of the targeting plasmid; and D. Chamovitz and R. Pardi for critical reading of the manuscript and discussions. Supported by the National Institutes of Health (R01-GM61812 to N.W., and R37-GM047850 to X.W.D.), the Arthritis Foundation (H.C.) and the Howard Hughes Medical Institute (R.A.F.).
Author information
Authors and Affiliations
Contributions
S.M. and H.C. designed and did T cell and MEF experiments; H.Z. and S.M. did Chromatin IP. S.M. together with the technical staff in R.A.F. lab generated the knockout strain. S.M. and N.W. bred mouse colonies and characterized the embryos. H.C., S.M. and N.W. analyzed data and wrote the manuscript; N.W. R.A.F. and X.W.D. provided advice and overall direction. The laboratories of N.W. and R.A.F. contributed equally to this study.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9, Tables 1–2, Methods (PDF 1760 kb)
Rights and permissions
About this article
Cite this article
Menon, S., Chi, H., Zhang, H. et al. COP9 signalosome subunit 8 is essential for peripheral T cell homeostasis and antigen receptor–induced entry into the cell cycle from quiescence. Nat Immunol 8, 1236–1245 (2007). https://doi.org/10.1038/ni1514
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1514
This article is cited by
-
Knockdown of subunit 3 of the COP9 signalosome inhibits C2C12 myoblast differentiation via NF-KappaB signaling pathway
BMC Pharmacology and Toxicology (2017)
-
Comparative muscle proteomics/phosphoproteomics analysis provides new insight for the biosafety evaluation of fat-1 transgenic cattle
Transgenic Research (2017)
-
COP9 signalosome subunit PfCsnE regulates secondary metabolism and conidial formation in Pestalotiopsis fici
Science China Life Sciences (2017)
-
Cops2 promotes pluripotency maintenance by Stabilizing Nanog Protein and Repressing Transcription
Scientific Reports (2016)
-
DCAF1 controls T-cell function via p53-dependent and -independent mechanisms
Nature Communications (2016)