Abstract
Positive selection occurs in the thymic cortex, but critical maturation events occur later in the medulla. Here we defined the precise stage at which T cells acquired competence to proliferate and emigrate. Transcriptome analysis of late gene changes suggested roles for the transcription factor NF-κB and interferon signaling. Mice lacking the inhibitor of NF-κB (IκB) kinase (IKK) kinase TAK1 underwent normal positive selection but exhibited a specific block in functional maturation. NF-κB signaling provided protection from death mediated by the cytokine TNF and was required for proliferation and emigration. The interferon signature was independent of NF-κB; however, thymocytes deficient in the interferon-α (IFN-α) receptor IFN-αR showed reduced expression of the transcription factor STAT1 and phenotypic abnormality but were able to proliferate. Thus, both NF-κB and tonic interferon signals are involved in the final maturation of thymocytes into naive T cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Huang, Y.H., Li, D., Winoto, A. & Robey, E.A. Distinct transcriptional programs in thymocytes responding to T cell receptor, Notch, and positive selection signals. Proc. Natl. Acad. Sci. USA 101, 4936–4941 (2004).
Mick, V.E., Starr, T.K., McCaughtry, T.M., McNeil, L.K. & Hogquist, K.A. The regulated expression of a diverse set of genes during thymocyte positive selection in vivo. J. Immunol. 173, 5434–5444 (2004).
Ueno, T. et al. CCR7 signals are essential for cortex-medulla migration of developing thymocytes. J. Exp. Med. 200, 493–505 (2004).
Setoguchi, R. et al. Repression of the transcription factor Th-POK by Runx complexes in cytotoxic T cell development. Science 319, 822–825 (2008).
He, X. et al. CD4-CD8 lineage commitment is regulated by a silencer element at the ThPOK transcription-factor locus. Immunity 28, 346–358 (2008).
Kishimoto, H. & Sprent, J. Negative selection in the thymus includes semimature T cells. J. Exp. Med. 185, 263–271 (1997).
Villunger, A. et al. Negative selection of semimature CD4+8−HSA+ thymocytes requires the BH3-only protein Bim but is independent of death receptor signaling. Proc. Natl. Acad. Sci. USA 101, 7052–7057 (2004).
Cowan, J.E. et al. The thymic medulla is required for Foxp3+ regulatory but not conventional CD4+ thymocyte development. J. Exp. Med. 210, 675–681 (2013).
Li, J. et al. Developmental pathway of CD4+CD8− medullary thymocytes during mouse ontogeny and its defect in Aire-/- mice. Proc. Natl. Acad. Sci. USA 104, 18175–18180 (2007).
McCaughtry, T.M., Wilken, M.S. & Hogquist, K.A. Thymic emigration revisited. J. Exp. Med. 204, 2513–2520 (2007).
Cowan, J.E. et al. Differential requirement for CCR4 and CCR7 during the development of innate and adaptive αβT cells in the adult thymus. J. Immunol. 193, 1204–1212 (2014).
Boursalian, T.E., Golob, J., Soper, D.M., Cooper, C.J. & Fink, P.J. Continued maturation of thymic emigrants in the periphery. Nat. Immunol. 5, 418–425 (2004).
Mingueneau, M. et al. Immunological Genome Consortium. The transcriptional landscape of αβ T cell differentiation. Nat. Immunol. 14, 619–632 (2013).
Singer, A., Adoro, S. & Park, J.H. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat. Rev. Immunol. 8, 788–801 (2008).
Matloubian, M. et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004).
Arbonés, M.L. et al. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity 1, 247–260 (1994).
Carlson, C.M. et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature 442, 299–302 (2006).
Priyadharshini, B., Welsh, R.M., Greiner, D.L., Gerstein, R.M. & Brehm, M.A. Maturation-dependent licensing of naive T cells for rapid TNF production. PLoS One 5, e15038 (2010).
Hoffmann, R. & Melchers, F. A genomic view of lymphocyte development. Curr. Opin. Immunol. 15, 239–245 (2003).
Ramsdell, F., Jenkins, M., Dinh, Q. & Fowlkes, B.J. The majority of CD4+8− thymocytes are functionally immature. J. Immunol. 147, 1779–1785 (1991).
Aliahmad, P. & Kaye, J. Commitment issues: linking positive selection signals and lineage diversification in the thymus. Immunol. Rev. 209, 253–273 (2006).
Carpenter, A.C. & Bosselut, R. Decision checkpoints in the thymus. Nat. Immunol. 11, 666–673 (2010).
Hogquist, K.A., Xing, Y., Hsu, F.C. & Shapiro, V.S. T Cell Adolescence: Maturation Events Beyond Positive Selection. J. Immunol. 195, 1351–1357 (2015).
Sato, S. et al. TAK1 is indispensable for development of T cells and prevention of colitis by the generation of regulatory T cells. Int. Immunol. 18, 1405–1411 (2006).
Kurobe, H. et al. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity 24, 165–177 (2006).
Wan, Y.Y., Chi, H., Xie, M., Schneider, M.D. & Flavell, R.A. The kinase TAK1 integrates antigen and cytokine receptor signaling for T cell development, survival and function. Nat. Immunol. 7, 851–858 (2006).
Liu, Z.G., Hsu, H., Goeddel, D.V. & Karin, M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell 87, 565–576 (1996).
Adhikari, A., Xu, M. & Chen, Z.J. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene 26, 3214–3226 (2007).
Jimi, E., Strickland, I., Voll, R.E., Long, M. & Ghosh, S. Differential role of the transcription factor NF-κB in selection and survival of CD4+ and CD8+ thymocytes. Immunity 29, 523–537 (2008).
Otero, D.C., Baker, D.P. & David, M. IRF7-dependent IFN-β production in response to RANKL promotes medullary thymic epithelial cell development. J. Immunol. 190, 3289–3298 (2013).
Lienenklaus, S. et al. Novel reporter mouse reveals constitutive and inflammatory expression of IFN-β in vivo. J. Immunol. 183, 3229–3236 (2009).
Gough, D.J. et al. Functional crosstalk between type I and II interferon through the regulated expression of STAT1. PLoS Biol. 8, e1000361 (2010).
Hata, N. et al. Constitutive IFN-α/β signal for efficient IFN-α/β gene induction by virus. Biochem. Biophys. Res. Commun. 285, 518–525 (2001).
Gough, D.J., Messina, N.L., Clarke, C.J., Johnstone, R.W. & Levy, D.E. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity 36, 166–174 (2012).
Dong, J. et al. Homeostatic properties and phenotypic maturation of murine CD4+ pre-thymic emigrants in the thymus. PLoS One 8, e56378 (2013).
Abt, M.C. et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 37, 158–170 (2012).
Kawashima, T. et al. Double-stranded RNA of intestinal commensal but not pathogenic bacteria triggers production of protective interferon-β. Immunity 38, 1187–1197 (2013).
Ganal, S.C. et al. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity 37, 171–186 (2012).
Yu, Q. et al. DNA-damage-induced type I interferon promotes senescence and inhibits stem cell function. Cell Rep. 11, 785–797 (2015).
Härtlova, A. et al. DNA damage primes the type I interferon system via the cytosolic DNA sensor STING to promote anti-microbial innate immunity. Immunity 42, 332–343 (2015).
Metidji, A. et al. IFN-α/β receptor signaling promotes regulatory T cell development and function under stress conditions. J. Immunol. 194, 4265–4276 (2015).
Silke, J. The regulation of TNF signalling: what a tangled web we weave. Curr. Opin. Immunol. 23, 620–626 (2011).
Zhang, N. & He, Y.W. An essential role for c-FLIP in the efficient development of mature T lymphocytes. J. Exp. Med. 202, 395–404 (2005).
Jost, P.J. et al. Bcl10/Malt1 signaling is essential for TCR-induced NF-κB activation in thymocytes but dispensable for positive or negative selection. J. Immunol. 178, 953–960 (2007).
Molinero, L.L. et al. CARMA1 controls an early checkpoint in the thymic development of FoxP3+ regulatory T cells. J. Immunol. 182, 6736–6743 (2009).
Barnes, M.J. et al. Commitment to the regulatory T cell lineage requires CARMA1 in the thymus but not in the periphery. PLoS Biol. 7, e51 (2009).
Sinclair, C. & Seddon, B. Overlapping and asymmetric functions of TCR signaling during thymic selection of CD4 and CD8 lineages. J. Immunol. 192, 5151–5159 (2014).
Chen, W. & Konkel, J.E. Development of thymic Foxp3+ regulatory T cells: TGF-β matters. Eur. J. Immunol. 45, 958–965 (2015).
Rubtsov, Y.P. & Rudensky, A.Y. TGFβ signalling in control of T-cell-mediated self-reactivity. Nat. Rev. Immunol. 7, 443–453 (2007).
Mahmud, S.A. et al. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat. Immunol. 15, 473–481 (2014).
Weinreich, M.A. et al. KLF2 transcription-factor deficiency in T cells results in unrestrained cytokine production and upregulation of bystander chemokine receptors. Immunity 31, 122–130 (2009).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Mootha, V.K. et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 (2003).
Acknowledgements
We thank H. Chi (St. Jude Children's Hospital) for Rag2GFP mice; M. Mescher (University of Minnesota) for Ifnar1−/− mice; A. Strasser (Walter and Eliza Hall Institute) for Bcl2l11−/− mice; K. Hayakawa (Fox Chase Cancer Center) for monoclonal antibody SM6C10; and M.A. Farrar for comments and review of the manuscript. Supported by the NIH (R01 AI088209 and P01 AI35296 to K.A.H.).
Author information
Authors and Affiliations
Contributions
Y.X. designed and performed experiments, analyzed data, and wrote the manuscript; X.W. performed experiments and analyzed data; S.C.J. provided reagents, animals and input for the preparation of the manuscript; and K.A.H. directed the research, analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Comparison of staining methods for SP thymocyte subsets.
(a) Flow cytometric analysis of thymocytes from Rag2GFP mice (n=4). Gating strategy for exclusion of re-circulating mature T cells (GFP−), γδ T cells (GL3+), iNKT (CD1dTet+) and Treg (CD25+) cells. (b) Flow cytometric analysis of thymocytes from H2-Ab1−/− mice (n=3). (c) Flow cytometric analysis of CCR7+TCRβ+ thymocytes from Klf2GFP mice (n=3). (d) Comparison of staining methods for CD4SP and CD8SP thymocyte subsets from Rag2GFP mice (n=3).
Supplementary Figure 2 Gene-expression changes during thymocyte maturation.
(a) Dump gates for cell sorting using Rag2GFP mice (n=3). Red numbers indicate frequency of cells in adjacent gates among total CD4SP thymocytes. (b) The numbers of genes whose expression changes in MHC class II restricted T cells during positive selection and maturation. Gene expression changes ≥ 2 fold, with P ≥ 0.05 (unpaired, t-test, n=3)
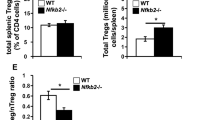
Supplementary Figure 3 A TAK1 signaling model, and ‘agonist-selected’ populations strongly affected by TAK1 deficiency.
(a) A schematic model showing TAK1 signaling transduction pathway components that potentially regulate T cell functional maturation. (b) Flow cytometric analysis and cell numbers of iNKT cells, Treg cells, IEL precursor (IELp) thymocytes and CD8aa gut intraepithelial lymphocytes (IEL) in Tak1fl/fl (n=5) and Tak1fl/flCd4Cre mice (n=5). Error bars indicate the mean ± s.d.; * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001, two-tailed unpaired Student’s t-test.
Supplementary Figure 4 Ingenuity pathway analysis of thymocytes.
Ingenuity pathway analysis defined upstream regulators of genes expressed differentially in “Early”, “Late” changed genes, and TAK1 dependent genes. Upstream regulators with p-values < 1.0E-08 are shown. Activation z-scores > 2.0 or < -2.0 are considered evidence of “Activated” or “Inhibited” pathways. Yellow highlighting indicated upstream regulators activated late in maturation that are lost with TAK1 deficiency. Likewise green highlighting indicates regulators that are inhibited late in maturation, but gained with TAK1 deficiency.
Supplementary Figure 5 Expression of genes encoding members of the TNFRSF superfamily during thymocyte maturation.
(a) Graphs show microarray gene expression signal values for all TNF receptor super family members (Tnfrsf) in each of the four populations indicated. (b) Protein expression of TNF receptor super family members was assessed by flow cytometry. Representative data from two independent experiments are shown. (c) Flow cytometric analysis of thymocytes from Tak1fl/fl, Tak1fl/flCd4Cre and Tak1fl/flCd4Cre Bcl2l11−/− mice. (d) Cell numbers of total thymocytes or indicated populations from Tak1fl/fl (n=10), Tak1fl/flCd4Cre (n=10) or Tak1fl/flCd4CreBcl2l11−/− mice (n=3). Error bars indicate the mean values ± s.d. in d. NS, not significant; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001 (unpaired t-test).
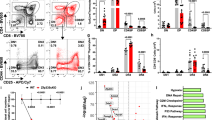
Supplementary Figure 6 IFN-αR is dispensable for TNF licensing and the proliferation competency of SP thymocytes.
(a) Flow cytometric analysis of TNF production of M2 CD4SP cells from Ifnar1−/− (n=3) and control mice (n=3). The cells were stimulated with plate-coated anti-CD3 plus anti-CD28 for 4 hours. Numbers indicate frequency of cells in adjacent gates and all plots have the same axis scales. (b) CellTrace Violet cell proliferation analysis of SM, M1 and M2 CD4SP cells from the indicated mice. Those cells were labeled with CellTrace Violet and then stimulated via CD3 and CD28 for 3 days. Representative data from three independent experiments are shown.
Supplementary information
Supplementary Text and Figures
Supplementary figures 1–6 and Supplementary Table 1 (PDF 1295 kb)
Rights and permissions
About this article
Cite this article
Xing, Y., Wang, X., Jameson, S. et al. Late stages of T cell maturation in the thymus involve NF-κB and tonic type I interferon signaling. Nat Immunol 17, 565–573 (2016). https://doi.org/10.1038/ni.3419
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3419
This article is cited by
-
A guide to thymic selection of T cells
Nature Reviews Immunology (2024)
-
The transcription factor LRF promotes integrin β7 expression by and gut homing of CD8αα+ intraepithelial lymphocyte precursors
Nature Immunology (2022)
-
TCF1 in T cell immunity: a broadened frontier
Nature Reviews Immunology (2022)
-
Screening of organoids derived from patients with breast cancer implicates the repressor NCOR2 in cytotoxic stress response and antitumor immunity
Nature Cancer (2022)
-
Identification of distinct functional thymic programming of fetal and pediatric human γδ thymocytes via single-cell analysis
Nature Communications (2022)