Abstract
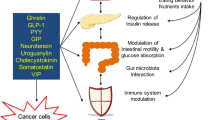
The obesity epidemic is fast becoming one of the leading causes of mortality and morbidity worldwide. Over the past 30 years, gastrointestinal hormones have been increasingly understood to have an important role as regulators of appetite and energy balance in obese individuals. The levels of these hormones are modulated by bariatric surgery, and understanding how they are affected by such procedures can contribute to our comprehension of the underlying mechanisms by which these hormones affect obesity and its treatment. In this Review, we consider several gastrointestinal hormones that can contribute to obesity by modulating the activity of the gut–brain axis, and examine their specific effects on appetite, hunger and energy balance. Better understanding of the mechanisms by which these peptides exert their effects may enable the development of improved weight-loss medications and new treatments for obesity.
Key Points
-
The obesity epidemic has highlighted the importance of understanding the mechanisms governing appetite and weight regulation
-
Over the past 30 years, gastrointestinal hormones have been found to have an integrated role in appetite regulation via their action on the 'gut–brain axis'
-
Bariatric surgery can achieve sustained weight loss in morbidly obese individuals and has been shown to modulate the levels of gastrointestinal hormones
-
Understanding the underlying mechanism of action of gastrointestinal hormones could provide useful insights into weight regulation, and contribute to the development of treatments for obesity
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
WHO (2004) Obesity; preventing and managing the global epidemic. Report of a WHO consultation on obesity. Geneva, Switzerland
Banks WA (1980) Evidence for a cholecystokinin gut–brain axis with modulation by bombesin. Peptides 1: 347–351
Schwartz MW et al. (2000) Central nervous system control of food intake. Nature 404: 661–671
Cone RD et al. (2001) The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord 25: S63–S67
Larsen PJ and Kristensen P (1997) The neuropeptide Y (Y4) receptor is highly expressed in neurones of the rat dorsal vagal complex. Brain Res Mol Brain Res 48: 1–6
Balasubramaniam A et al. (2006) Neuropeptide Y (NPY) Y4 receptor selective agonists based on NPY (32–36): development of an anorectic Y4 receptor selective agonist with picomolar affinity. J Med Chem 49: 2661–2665
Flier JS (2004) Obesity wars: molecular progress confronts an expanding epidemic. Cell 116: 337–350
Grill HJ and Smith GP (1988) Cholecystokinin decreases sucrose intake in chronic decerebrate rats. Am J Physiol 254: R853–R856
Ellacott KL et al. (2006) Interactions between gut peptides and the central melanocortin system in the regulation of energy homeostasis. Peptides 27: 340–349
Schwartz MW et al. (2003) Is the energy homeostasis system inherently biased toward weight gain? Diabetes 52: 232–238
Brady LS et al. (1990) Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food-deprived rats. Neuroendocrinology 52: 441–447
Leibel RL et al. (1995) Changes in energy expenditure resulting from altered body weight. N Engl J Med 332: 621–628
Gibbs J et al. (1973) Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol 84: 488–495
Murphy KG and Bloom SR (2006) Gut hormones and the regulation of energy homeostasis. Nature 444: 854–859
Murakami N et al. (2002) Role for central ghrelin in food intake and secretion profile of stomach ghrelin in rats. J Endocrinol 174: 283–288
Tschop M et al. (2000) Ghrelin induces adiposity in rodents. Nature 407: 194–198
Kojima M et al. (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402: 656–660
Baldanzi G et al. (2002) Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol 159: 1029–1037
Nakazato M et al. (2001) A role for ghrelin in the central regulation of feeding. Nature 409: 194–198
Wren AM et al. (2000) The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 141: 4325–4328
Cowley MA et al. (2003) The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37: 649–661
le Roux CW et al. (2005) Ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy. J Clin Endocrinol Metab 90: 4521–4524
Theander-Carrillo C et al. (2006) Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest 116: 1983–1993
Patel AD et al. (2006) Ghrelin stimulates insulin induced glucose uptake in adipocytes. Regul Pept 134: 17–22
Cummings DE et al. (2001) A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50: 1714–1719
Murray CD et al. (2006) The effect of different macronutrient infusions on appetite, ghrelin and peptide YY in parenterally fed patients. Clin Nutr 25: 626–633
Feinle-Bisset C et al. (2005) Fat digestion is required for the suppression of ghrelin and stimulation of peptide YY and pancreatic polypeptide secretion by intraduodenal lipid. Am J Physiol. Endocrinol Metab 289: E948–5E953
Cummings DE et al. (2002) Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 346: 1623–1630
Wren AM et al. (2001) Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 86: 5992
Druce MR et al. (2005) Ghrelin increases food intake in obese as well as lean subjects. Int J Obes Relat Metab Disord 29: 1130–1136
English PJ et al. (2002) Food fails to suppress ghrelin levels in obese humans. J Clin Endocrinol Metab 87: 2984
le Roux CW et al. (2005) Postprandial plasma ghrelin is suppressed proportional to meal calorie content in normal-weight but not obese subjects. J Clin Endocrinol Metab 90: 1068–1071
Sun Y et al. (2004) Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA 101: 4679–4684
Zigman JM et al. (2005) Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest 115: 3564–3572
Wortley KE et al. (2005) Absence of ghrelin protects against early onset obesity. J Clin Invest 115: 3573–3578
Wortley KE et al. (2004) Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc Natl Acad Sci USA 101: 8227–8232
Zhang JV et al. (2005) Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin's effect on food intake. Science 310: 996–999
Nogueiras R et al. (2007) effects of obestatin on energy balance and growth hormone secretions in rodents. Endocrinology 148: 21–26
Seoane LM et al. (2006) Central obestatin administration does not modify either spontaneous or ghrelin-induced food intake in rats. J Endocrinol Invest 29: RC13–RC15
Holst B et al. (2007) GPR39 signaling is stimulated by zinc ions but not by obestatin. Endocrinology 148: 13–20
Jackson VR et al. (2006) GPR39 receptor expression in the mouse brain. Neuroreport 17: 813–816
Gourcerol G et al. (2007) Lack of obestatin effects on food intake: should obestatin be renamed ghrelin-associated peptide (GAP)? Reg. Pept 141: 1–7
Batterham RL et al. (2003) Pancreatic polypeptide reduces appetite and food intake in humans. J Clin Endocrinol Metab 88: 3989–3992
Clark JT et al. (1984) Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology 115: 427–429
Whitcomb DC et al. (1997) Distribution of pancreatic polypeptide receptors in the rat brain. Brain Res 760: 137–149
Ueno N et al. (1999) Decreased food intake and body weight in pancreatic polypeptide-overexpressing mice. Gastroenterology 117: 1427–1432
Liddle RA et al. (1985) Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest 75: 1144–1152
Dockray GJ (1987) Peptides of the gut and brain: the cholecystokinins. Proc Nutr Soc 46: 119–124
Moran TH (2000) Cholecystokinin and satiety: current perspectives. Nutrition 16: 858–865
West DB et al. (1987) Lithium chloride, cholecystokinin and meal patterns: evidence that cholecystokinin suppresses meal size in rats without causing malaise. Appetite 8: 221–227
Asin KE et al. (1992) Effects of selective CCK receptor agonists on food intake after central or pheripheral administration in rats. Brain Res 571: 169–174
Dockray G (2004) Gut endocrine secretions and their relevance to satiety. Curr Opin Pharmacol 4: 557–560
Moran TH et al. (1998) Disordered food intake and obesity in rats lacking cholecystokinin A receptors. Am J Physiol 274: R618–R625
Beglinger C et al. (2001) Loxiglumide, a CCK-A receptor antagonist, stimulates calorie intake and hunger feelings in humans. Am J Physiol Regul Integr Comp Physiol 280: R1149–R1154
West DB et al. (1984) Cholecystokinin persistently suppresses meal size but not food intake in free-feeding rats. Am J Physiol 246: R776–R787
Crawley JN and Beinfeld MC (1983) Rapid development of tolerance to the behavioural actions of cholecystokinin. Nature 302: 703–706
Baggio LL and Drucker DJ (2007) Biology of incretins: GLP-1 and GIP. Gastroenterology 132: 2131–2157
Creutzfeldt W et al. (1978) Gastric inhibitory polypeptide (GIP) and insulin in obesity: increased response to stimulation and defective feedback control of serum levels. Diabetologia 14: 15–24
Salera M et al. (1982) Gastric inhibitory polypeptide release after oral glucose: relationship to glucose tolerance, diabetes mellitus and obesity. J Clin Endocrinol Metab 55: 329–336
Miyawaki K et al. (2002) Inhibition of gastric inhibitory polypeptide signalling prevents obesity. Nat Med 8: 738–742
Ding WG and Gromada J (1997) Protein Kinase A-dependent stimulation of exocytosis in mouse pancreatic beta-cells by glucose-dependent insulinotropic polypeptide. Diabetes 46: 615–621
Lacroix A et al. (1992) Gastric inhibitory polypeptide-dependent cortisol hypersecretion-a new cause of Cushing's syndrome. N Engl J Med 327: 974–980
Holst JJ (2004) On the physiology of GIP and GLP-1. Horm Metab Res 36: 747–754
Larsen PJ et al. (1997) Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology 138: 4445–4455
Abbott CR et al. (2005) The inhibitory effects of peripheral administration of peptide YY (3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstemhypothalamic pathway. Brain Res 1044 127–131
Verdich C et al. (2001) The role of postprandial releases of insulin and incretin hormones in meal-induced satiety-effect of obesity and weight reduction. Int J Obes Relat Metab Disord 25: 1206–1214
Fukase N et al. (1993) Hypersecretion of truncated glucagon-like peptide-1 and gastric inhibitory polypeptide in obese patients. Diabet Med 10: 44–49
Feinle C et al. (2002) Plasma glucagon-like peptide-1 (GLP-1) responses to duodenal fat and glucose infusions in lean and obese men. Peptides 23: 1491–1495
Kreymann B et al. (1987) Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet 2: 1300–1304
Edwards CM et al. (1999) Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9–39. Diabetes 48: 86–93
Gutzwiller JP et al. (1999) Glucagonlike peptide-1: a potent regulator of food intake in humans. Gut 44: 81–86
Edwards CM et al. (2001) Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol. Endocrinol Metab 281: E155–E161
Turton MD et al. (1996) A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379: 69–72
O'Shea D et al. (1996) A role for central glucagon-like peptide-1 in temperature regulation. Neuroreport 7: 830–832
Ruiz-Grande C et al. (1992) Lipolytic action of glucagons-like peptides in isolated rat adipocytes. Peptides 13: 13–16
Villanueva-Penacarrillo ML et al. (2001) Effect of GLP-1 on lipid metabolism in human adipocytes. Horm Metab Res 33: 73–77
Todd JF et al. (1998) Subcutaneous glucagon-like peptide-1 improves postprandial glycaemic control over a 3-week period in patients with early type 2 diabetes. Clin Sci (Lond) 95: 325–329
Kolterman OG et al. (2003) Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 88: 3082–3089
Soltani N et al. (2007) In vivo expression of GLP-1/IgG-Fc fusion protein enhances beta-cell mass and protects against streptozotocin-induced diabetes. Gene Ther 14: 981–988
DeFronzo RA et al. (2005) Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 28: 1092–1100
Kendall DM et al. (2005) Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 28: 1083–1091
Cohen MA et al. (2003) Oxyntomodulin suppresses appetite and reduces food intake in humans. J Clin Endocrinol Metab 88: 4696–4701
Dakin CL et al. (2004) Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology 145: 2687–2695
Baggio LL et al. (2004) Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology 127: 546–558
Born W et al. (2002) Receptors for calcitonin gene-related peptide, adrenomedullin, and amylin: the contributions of novel receptor-activity-modifying proteins. Receptors Channels 8: 201–209
Wynne K et al. (2005) Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes 54: 2390–2395
Wynne K et al. (2006) Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. Int J Obes (Lond) 30: 1729–1736
Tatemoto K and Mutt V (1980) Isolation of two novel candidate hormones using a chemical method for finding naturally occurring polypeptides. Nature 285: 417–418
Adrian TE et al. (1985) Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 89: 1070–1077
Ali-Rachedi A et al. (1984) Peptide YY (PYY) immunoreactivity is co-stored with glucagon-related immunoreactants in endocrine cells of the gut and pancreas. Histochemistry 80: 487–491
Allen JM et al. (1984) Effects of peptide YY and neuropeptide Y on gastric emptying in man. Digestion 30: 255–262
Adrian TE et al. (1985) Effect of peptide YY on gastric, pancreatic, and biliary function in humans. Gastroenterology 89: 494–499
le Roux CW et al. (2006) Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 147: 3–8
Tschöp M et al. (2004) Physiology: does gut hormone PYY3-36 decrease food intake in rodents? Nature 430: 1 p following 165; discussion 2 p following 165
Degen L et al. (2005) Effect of peptide YY3–36 on food intake in humans. Gastroenterology 129: 1430–1436
Koegler FH et al. (205) Peptide YY(3–36) inhibits morning, but not evening, food intake and decreases body weight in rhesus macaques. Diabetes 54: 3198–3204
Chelikani PK et al. (2005) Intravenous infusion of peptide YY (3–36) potently inhibits food intake in rats. Endocrinology 146: 879–888
Boey D et al. (2006) Peptide YY ablation in mice leads to the development of hyperinsulinaemia and obesity. Diabetologia 49: 1360–1370
Batterham RL et al. (2002) Gut hormone PYY (3–36) physiologically inhibits food intake. Nature 418: 650–654
Acuna-Goycolea C and van den Pol AN (2005) Peptide YY (3–36) inhibits both anorexigenic proopiomelanocortin and orexigenic neuropeptide Y neurons: implications for hypothalamic regulation of energy homeostasis. J Neurosci 25: 10510–10519
Abbott CR et al. (2005) Blockade of the neuropeptide YY2 receptor with the specific antagonist BIIE0246 attenuates the effect of endogenous and exogenous peptide YY(3–36) on food intake. Brain Res 1043: 139–144
Batterham RL et al. (2003) Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med 349: 941–948
Stock S et al. (2005) Ghrelin, peptide YY, glucose-dependent insulino-tropic polypeptide, and hunger responses to a mixed meal in anorexic, obese and control female adolescents. J Clin Endocrinol Metab 90: 2161–2168
Korner J et al. (2005) Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY and insulin. J Clin Endocrinol Metab 90: 359–365
Batterham RL et al. (2006) Critical role of peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab 4: 223–233
Halatchev IG and Cone RD (2005) Peripheral administration of PYY (3–36) produces conditioned taste aversion in mice. Cell Metab 1: 159–168
Talsania T et al. (2005) Peripheral exendin-4 and peptide YY (3–36) synergistically reduce food intake through different mechanisms in mice. Endocrinology 146: 3748–3756
Greenough A et al. (1998) Untangling the effects of hunger, anxiety, and nausea on energy intake during intravenous cholecystokinin octapeptide (CCK-8) infusion. Physiol Behav 65: 303–310
Naslund E et al. (2004) Prandial subcutaneous injections of glucagonlike peptide-1 cause weight loss in obese human subjects. Br J Nutr 91: 439–446
Adrian TE et al. (1986) Peptide YY abnormalities in gastrointestinal diseases. Gastroenterology 90: 379–384
Di Francesco V et al. (2005) Delayed postprandial gastric emptying and impaired gallbladder contraction together with elevated cholecystokinin and peptide YY serum levels sustain satiety and inhibit hunger in healthy elderly persons. J Gerontol A Biol Sci Med Sci 60: 1581–1585
Sjöström L et al. (2007) Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357: 741–752
Sjöström L et al. (2004) Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351: 2683–2693
Borg CM et al. (2006) Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg 93: 210–215
le Roux CW et al. (2007) Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 246: 780–785
Naslund E et al. (1997) Gastrointestinal hormones and gastric emptying 20 years after jejunoileal bypass for massive obesity. Int J Obes Relat Metab Disord 21: 387–392
Aylwin S (2005) Gastrointestinal surgery and gut hormones. Curr Opin Endocrinol. Diabetes 12: 89–98
Neary NM et al. (2005) Peptide YY3-36 and glucagon-like peptide-17-36 inhibit food intake additively. Endocrinology 146: 5120–5127
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Vincent, R., Ashrafian, H. & le Roux, C. Mechanisms of Disease: the role of gastrointestinal hormones in appetite and obesity. Nat Rev Gastroenterol Hepatol 5, 268–277 (2008). https://doi.org/10.1038/ncpgasthep1118
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpgasthep1118
This article is cited by
-
Acute consumption of a shake containing cashew and Brazil nuts did not affect appetite in overweight subjects: a randomized, cross-over study
European Journal of Nutrition (2021)
-
Relationship of Body Composition Measures and Metabolic Basal Rate with Gastrointestinal Hormones in Weight Regain 5 Years After Gastric Bypass
Obesity Surgery (2020)
-
The role of bariatric surgery to treat diabetes: current challenges and perspectives
BMC Endocrine Disorders (2017)
-
Gut Hormones and Leptin: Impact on Energy Control and Changes After Bariatric Surgery—What the Future Holds
Obesity Surgery (2012)
-
Body Image After Sleeve Gastrectomy: Reduced Dissatisfaction and Increased Dynamics
Obesity Surgery (2012)