Abstract
Hoogsteen (HG) base pairing involves a 180° rotation of the purine base relative to Watson–Crick (WC) base pairing within DNA duplexes, creating alternative DNA conformations that can play roles in recognition, damage induction and replication. Here, using nuclear magnetic resonance R1ρ relaxation dispersion, we show that transient HG base pairs occur across more diverse sequence and positional contexts than previously anticipated. We observe sequence-specific variations in HG base pair energetic stabilities that are comparable with variations in WC base pair stability, with HG base pairs being more abundant for energetically less favourable WC base pairs. Our results suggest that the variations in HG stabilities and rates of formation are dominated by variations in WC base pair stability, suggesting a late transition state for the WC-to-HG conformational switch. The occurrence of sequence and position-dependent HG base pairs provide a new potential mechanism for achieving sequence-dependent DNA transactions.
Similar content being viewed by others
Introduction
We recently showed1,2,3 using nuclear magnetic resonance (NMR) R1ρ relaxation dispersion (RD)4,5,6 that A·T and G·C Watson–Crick (WC) base pairs (bps) in CA/TG and TA/TA steps of duplex DNA transiently form Hoogsteen (HG) bps7 with populations ranging between 0.14 and 0.49% and lifetimes between 0.3 and 2.5 ms at pH ~6 (Fig. 1a). HG bps form through 180° rotation of the purine base around the glycosidic bond from an anti to syn conformation (Fig. 1a). HG bps modify the structural and chemical presentation of DNA and thereby can play unique roles (reviewed in ref. 8) in DNA–protein recognition9,10,11,12, damage induction13 and repair14,15 as well as replication16,17. For example, by narrowing the minor groove, HG bps have been shown to alter the DNA electrostatic potential ‘seen’ by DNA-binding proteins at the bp edges in the DNA grooves in the context of p53-DNA complexes12,18,19. HG bps can also transiently expose DNA sites that are otherwise inaccessible in WC bps, and thereby provide new mechanisms for damage induction. For example, using computational mapping, Bohnuud et al.13 recently showed that transient G·C+ HG bps could explain the susceptibility and accessibility of cytosines to hydroxymethylation by formaldehyde, thus explaining a long-standing mystery that has persisted for over 25 years. There is also structural and biochemical evidence that some members of the low-fidelity Y-family polymerases replicate DNA using HG pairing as the dominant mechanism, thus providing a mode for bypassing a variety of lesions on the WC face during replication16,17,20. Naked duplexes consisting entirely of HG bps have been reported in A–T-rich sequences that form parallel21 and anti-parallel stranded22,23 DNA. Computational studies on duplexes also suggest that the HG bp is a reasonable conformation, only slightly less stable than the canonical WC bp24,25.
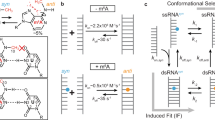
(a) The equilibrium between Watson–Crick and Hoogsteen base pairs. Shown are the average forward (kA) and reverse (kB) rate constants and populations of Watson–Crick (pA) and Hoogsteen (pB) base pairs obtained in this study. (b) DNA duplexes used in this study. Base pairs targeted for relaxation dispersion measurements are highlighted with a star. (c) Representative off-resonance 13C and 15N R1ρ relaxation dispersion profiles showing chemical exchange outside TA and CA steps of canonical duplex DNA. Spin lock powers (Hz) are shown in the inset. Data are fit to equation 2. Error bars represent experimental uncertainty (one standard deviation) estimated from monoexponential fitting of duplicate R1ρ data and analysis of signal-to-noise. See Methods for buffer conditions.
Considering that HG bps are an energetically favourable alternative to WC bps that can provide new mechanisms in a wide variety of DNA biochemical processes, it is of great interest to explore whether the transient HG bps observed by NMR are confined to flexible CA and TA steps, or occur more broadly across distinct sequence and position contexts in duplex DNA. Likewise, it is of interest to examine the sequence specificity of transient HG bp formation as this could provide new mechanisms for achieving sequence-specific DNA transactions that are based on shape19.
By carrying out 13C and 15N R1ρ NMR RD measurements on 33 bps in eight distinct canonical DNA duplexes, we show that transient HG bps are not limited to CA and TA steps, but rather occur broadly across diverse sequence and positional contexts. We find that both the energetic stability and rates of HG bp formation exhibit a dependence on sequence and position, with HG bps forming faster and being more abundant in energetically less favourable WC bps.
Results
Widespread transient HG bps in duplex DNA
To more broadly examine the occurrence and sequence specificity of transient HG bps in canonical duplex DNA, we carried out 13C and 15N R1ρ NMR RD measurements targeting sugar C1′ and base C6/8 or N1/3 resonances in 20 A·T and 13 G·C bps in eight DNA duplexes that encompass a variety of sequence motifs, including (A·T)n repeats of varying length (n=2, 4, 5 and 6), a (CA)3 repeat, a duplex sequence that forms HG bps on binding to the antibiotic echinomycin,1,26,27 a (CG)3 repeat capable of forming Z-DNA28, and a B/Z junction forming sequence29,30 (Fig. 1b). The targeted bps (Fig. 1b, highlighted in stars) encompass 6/10 dinucleotide steps that are positioned 1–6 bps away from the closest terminal end. R1ρ RD experiments were performed at pH 5.2–7.5 and 5.2–5.4 for A·T and G·C bps, respectively. We use low-pH conditions for transient (and protonated) G·C bps in order to increase the WC-to-HG chemical exchange signature detected by NMR R1ρ RD3. We recently reported a detailed analysis of the pH dependence of chemical exchange corresponding to transient HG bp formation and how measurements at such lower pH conditions can be qualitatively interpolated to assess exchange at higher pH3.
The R1ρ NMR RD experiment measures the line broadening contribution to resonances of interest due to chemical exchange with a transient, lowly populated species4,5. In all cases, we measured significant 13C and/or 15N R1ρ RD (Supplementary Table 1) consistent with chemical exchange (Fig. 1c and Supplementary Fig. 1). A two-state analysis  of the R1ρ data4,5,6 (see Methods) yielded populations (pB=~0.08–2.73%) and lifetimes (τB=~0.12–2.57 ms) for the transient state (Supplementary Fig. 2 and Supplementary Table 2) that are similar to those reported previously for transient HG bps (pB=~0.14–0.49% and τB=~0.3–2.5 ms)1,2. The chemical shifts (ωB) of the transient state obtained using this analysis (Supplementary Fig. 3 and Supplementary Table 2) are also consistent with HG bps, including significantly downfield shifted purine C8 (Δω≈2.72 p.p.m.), purine C1′ (Δω≈3.41 p.p.m.), cytosine C6 (Δω≈2.40 p.p.m.) and upfield shifted imino N1/3 (Δω≈−1.84 p.p.m.)1,2. Consistent with HG bps, we did not observe significant chemical exchange at adenine C2 and thymine C6, which do not experience large chemical shift changes upon HG bp formation1,2 (Supplementary Fig. 1 and Supplementary Table 2). These results show that transient HG bps are not confined to CA/TG and TA/TA steps but rather occur broadly across a wide variety of sequence and positional contexts, including GA/TC, AA/TT, TA/TA, GG/CC, CG/CG and TG/CA dinucleotide steps (where the HG bp is underlined).
of the R1ρ data4,5,6 (see Methods) yielded populations (pB=~0.08–2.73%) and lifetimes (τB=~0.12–2.57 ms) for the transient state (Supplementary Fig. 2 and Supplementary Table 2) that are similar to those reported previously for transient HG bps (pB=~0.14–0.49% and τB=~0.3–2.5 ms)1,2. The chemical shifts (ωB) of the transient state obtained using this analysis (Supplementary Fig. 3 and Supplementary Table 2) are also consistent with HG bps, including significantly downfield shifted purine C8 (Δω≈2.72 p.p.m.), purine C1′ (Δω≈3.41 p.p.m.), cytosine C6 (Δω≈2.40 p.p.m.) and upfield shifted imino N1/3 (Δω≈−1.84 p.p.m.)1,2. Consistent with HG bps, we did not observe significant chemical exchange at adenine C2 and thymine C6, which do not experience large chemical shift changes upon HG bp formation1,2 (Supplementary Fig. 1 and Supplementary Table 2). These results show that transient HG bps are not confined to CA/TG and TA/TA steps but rather occur broadly across a wide variety of sequence and positional contexts, including GA/TC, AA/TT, TA/TA, GG/CC, CG/CG and TG/CA dinucleotide steps (where the HG bp is underlined).
Position- and sequence-dependent energetic variability
We observe ~30-fold variations in the transient HG population (0.08–2.73% and 0.13–2.11% for A·T and G·C+ bps, respectively) and ~20-fold variations in lifetimes (0.12–2.57 ms and 0.40–2.08 ms for A·T and G·C+ bps, respectively) (Supplementary Fig. 2 and Supplementary Table 2). This corresponds to ~2.1 kcal mol−1 and ~2.8 kcal mol−1 variations in the relative thermodynamic stability (ΔΔGWC–HG with ΔGWC−HG=GHG−GWC) (Fig. 2a) and forward free-energy barriers (ΔΔG‡WC−HG with ΔG‡WC−HG=GTS−GWC, where TS is the transition state), respectively (Fig. 2b). These variations could reflect real sequence or position dependencies for transient HG bp formation. Alternatively, they could arise due to small differences in buffer conditions used for some of the duplexes (see Methods), particularly pH, which can affect the energetics of transient G·C+ HG bp formation1,2,3. However, systematic deviations in ΔGWC−HG and ΔG‡WC–HG are not observed across DNA duplexes (Supplementary Fig. 4a). Furthermore, no correlations are observed between duplex melting temperatures measured by circular dichroism (CD) (Supplementary Fig. 4b,c and Supplementary Table 3) and either ΔGWC−HG or ΔG‡WC−HG (Supplementary Fig. 4a). Consistent with sequence- and/or position-dependent contributions, the variations in ΔGWC−HG and ΔG‡WC−HG are smaller (~1 kcal mol−1) when comparing bps across different duplexes that share identical 5′ and 3′ neighbours and positions relative to duplex ends (indicated using horizontal lines in Fig. 2a,b; Supplementary Fig. 2 and Supplementary Table 2).
(a) Free-energy difference (ΔGWC−HG) and (b) forward free-energy barrier (ΔG‡WC−HG) for the Watson–Crick to Hoogsteen transition as derived from a two-state analysis of the R1ρ data. Error bars represent experimental uncertainty (one s.d.) estimated from propagation of errors from monoexponential fitting of duplicate R1ρ data and analysis of signal-to-noise. Average and standard deviations for dinucleotide steps are shown using white bars. Horizontal lines denote bps with same triplet sequence and positions relative to nearest terminal end. X axis labels denote the triple sequence context (‘XYZ’) with Hoogsteen base pair in the middle; the position relative to the closest terminal end (‘−n’), and name of duplex as denoted in Fig. 1b. Correlation between (c) ΔG‡WC−HG and ΔGWC−HG as well as (d) ΔG‡HG−WC and ΔGWC−HG. A·T and G·C+ base pairs are shown in red and blue, respectively. The best line of fit and corresponding Pearson coefficient (R) are shown. (e) Free-energy diagram of the Watson–Crick to Hoogsteen transition depicting relative variations in free energy of Watson–Crick, transition and Hoogsteen states.
We observe significant variations in ΔGWC−HG and ΔG‡WC−HG for the same dinucleotide step, which may arise due to differences in position and/or differences in the broader sequence context. Notwithstanding these variations, the average stabilities of HG bps relative to WC bps (ΔGWC−HG) calculated for individual dinucleotide steps (Fig. 2a) follow an order (TA/TA>AA/TT>CA/TG>GA/TC for A·T and TG/CA≥CG/CG≥GG/CC for G·C+) that is nearly inverted relative to the well-documented WC dinucleotide stabilities (GA/TC≥CA/TG>AA/TT>TA/TA for A·T and CG/CG>GG/CC>TG/CA for G·C), which measure the stability of WC bps relative to the melted state31. Thus, transient HG bps seem to be more abundant in less stable WC dinucleotide steps such as CA/TG and TA/TA steps. The energetic preference for HG bps at TA/TA steps observed here is consistent with a large body of data showing that HG bps are favoured in A–T-rich sequences, particularly TA/TA steps (reviewed in ref. 8).
Origin of variable transient HG bp energetics
Strikingly, we observe a clear correlation (R=0.76) between ΔGWC−HG and ΔG‡WC−HG (Fig. 2c) and a relatively uniform backward free-energy barrier (ΔG‡HG−WC=GTS−GHG) of ~13 kcal mol−1 (Fig. 2d). Thus, changing the sequence context has little effect on the relative energetic stability of the TS and HG bp. This could either be because the stabilities of the TS and HG bp are not significantly affected by changes in sequence, or because their stabilities vary in a correlated manner. In contrast, a change in sequence context does change the relative stability of both the TS and HG bp relative to the WC bp (Fig. 2e).
One possibility is that sequence-specific variations are dominated by changes in variations in the WC bp without significantly affecting the stabilities of the TS and HG bp (Fig. 2e). Indeed, the observed variations in HG bp stability (~2.1 kcal mol−1) are comparable in size with variations in WC bp stability (~2 kcal mol−1) measured across dinucleotide steps using melting experiments31. This would also explain why transient HG bps are specifically more abundant at dinucleotide steps that have weakened WC stabilities (Fig. 2a,b). If the observed variations are indeed dominated by variations in WC stability, one might expect a similar correlation between the ΔGcl−op and ΔG‡cl−op values describing transitions between WC bps and the bp open state, especially since stability of the open state is not expected to vary significantly with sequence. Indeed, a previous analysis of ΔGcl−op and ΔG‡cl−op correlation reported by Coman and Russu32 based on imino proton exchange measurements33 reveals a comparably strong correlation (R=0.8)34. Our results suggest that the free energy of the TS varies less relative to the HG bp with sequence/position as compared with the WC bp. If one were to assume that a similar sequence/position-dependent free energy implies a similar structure, even if the sequence/position dependence is very small, then these results would suggest that the TS is structurally more similar to the HG bp—consistent with a ‘late’ TS.
Φ-value analysis suggests a ‘late’ transition state
To quantify the extent to which the sequence-specific TS energetics are more similar to HG bps versus WC bps, we subjected the measured ΔGWC−HG and ΔG‡WC−HG values to Φ-value analysis34,35 (Methods and Fig. 3). In this approach, one computes a Φ-value, which quantifies the relative magnitude of the sequence/position-dependent free-energy differences between the TS and WC bps and those between WC bps and HG bps,
Perturbing the Watson–Crick to Hoogsteen equilibrium in (a) A·T and (b) G·C+ base pairs by varying the sequence and/or positional context of individual base pairs. All duplexes are re-drawn after aligning each A·T or G·C+ base pair with a reference sequence variant (Ψ−WT). (c) Φ-values for A·T and G·C+ base pairs. The sequence variant number is shown above each secondary structure.

where ΔΔGTS−WC=(GTS−GWC)mut−(GTS−GWC)Ψ−WT and ΔΔGWC−HG=(GHG−GWC)mut−(GHG−GWC)Ψ−WT are the changes in the forward free-energy barrier and free-energy difference between WC and HG bps, respectively, on mutating (mut) the sequence/position of a reference (Ψ−WT) bp. It is instructive to consider two limiting cases to help understand how this analysis can be used to quantify the extent to which the sequence-specific TS energetics are more similar to HG bps versus WC bps and whether a TS is ‘early’ or ‘late’. In the case that the TS and HG share identical sequence-specific energetics as might be expected for a late TS, a given sequence-specific perturbation equally affects GTS and GHG (that is, GHG−GTS=constant for all mutants) and ΔΔGTS−WC=ΔΔGWC−HG and Φ=1. On the other hand, if TS and WC share identical sequence-specific energetics as might be expected for an early TS, then GTS–GWC=constant for all mutants and ΔΔGTS−WC=0 and Φ=0. In practice, the Φ-value can range between 0 and 1, with intermediate Φ-values being more difficult to interpret in the context of a structural mechanism14.
We arbitrarily assign reference A·T and G·C bps to be those having the smallest ΔGWC−HG values, and which therefore form the most stable HG bps among those studied herein (Ψ−WT, Fig. 3a,b). Next, we computed Φ for each A·T and G·C+ bp. This analysis was preformed separately for A·T and G·C+ bps and repeated multiple times assuming a different bp as the designated wild-type reference (data not shown). In the vast majority of the cases, we measure Φ-values near 1, consistent with a ‘late’ TS (Fig. 3c and Supplementary Table 4). It should be noted that similar sequence/position-dependent energetics for the TS and HG bp does not have to imply similarity in structure, and that we cannot rule out the possibility of an early TS that has structural features similar to WC but sequence/position-dependent energetics that are more similar to HG.
Discussion
Our results suggest that HG bps can occur ubiquitously in canonical duplex DNA across different sequence and positional contexts. This can help explain how polymerases such as the human DNA polymerase iota can use HG pairing as a general mechanism to replicate DNA and thereby bypass lesions that diminish the ability to form WC bps8,16. Our findings also raise the possibility that HG bps may be widespread in genomic DNA, especially given that the energetic differences between WC and HG bps are small compared with forces in living cells, including those arising due to supercoiling, torsional stress, and protein binding. It is worth noting that difficulties in distinguishing between WC and HG bps based on X-ray crystallography data have been reported8,36. Our finding that transient HG bps can occur ubiquitously in duplex DNA calls for the re-examination of current X-ray structures of DNA to more critically assess for the occurrence of HG bps.
Our studies suggest that the occurrence of HG bps depends in a complex manner on both sequence context and position. This is consistent with the hypothesis by Honig and Rohs37 that the observation of HG base pairing makes it is unlikely that protein–DNA binding is driven by a simple linear code. Nevertheless, our results together with prior studies8 suggest that HG bps are likely to exist in greater abundance within unstable and structurally stressed environments, such as kinks and turns, which can destabilize WC bps, including stacking interactions. Indeed, while only a few X-ray structures have documented the existence of HG bps in DNA, in many cases HG bps occur near structurally stressed environments. For example, HG bps are observed near kinks or nicks in X-ray structures of DNA bound to TATA box-binding protein11 and integration host factor9, and near a hairpin loop in structures of DNA bound to TnpA transposase38. HG bps have also been observed for DNA in complex with antibiotics that contribute unique stacking interactions27,39. Previous studies3 have shown that changes in counterion concentration have a measurable effect on the population and lifetime of HG bps. Future studies should therefore also examine how the sequence- and position-dependent HG energetics vary with increasing counterion concentration (Na+ and Mg2+). Studies so far suggest that Na+ and Mg2+ stabilize A·T HG but destabilize G·C+ HG bps in the case of CA/TG steps3. Although further studies are needed to more quantitatively understand the sequence and position dependence of transient HG bp formation, these energetic preferences may provide a new mechanism for shape-based DNA recognition12,19 via indirect read-out mechanisms40. Our study has focused on the occurrence of single transient HG bps surrounded by WC bps. Additional studies are needed to examine sequence-specific propensities for forming longer stretches of HG bps, and HG tracts interspersed by WC bps. Such mixtures of WC and HG bps can endow genomic DNA with a new level of structural complexity similar to Z-DNA.
Our results suggest that the sequence- and position-specific variations in HG bp stabilities and lifetimes are dominated by variations in the WC bp stability and to a lesser extent by variations in the stabilities of the TS and HG bp. Interestingly, a similar trend has been reported for base opening32. Future studies should further explore the WC-to-HG transition pathways and examine whether they share a similar TS with base opening and whether there can be pathways toward HG that proceed via the base-opened state. Conjugate peak refinement simulations suggest a pathway in which the purine base rotates toward the major groove inside the double helix1; however, further experimental characterization is required. The observation that the sequence and/or position variations in the TS free energies are more similar to the HG bp versus the WC bp suggests the TS is structurally more similar to HG versus WC, consistent with a ‘late’ TS for the WC-to-HG transition. However, we cannot rule out an early TS that is structurally more similar to WC but has sequence/position-dependent energetics that are more similar to the HG bp. Although the structure of the TS remains unclear, an equally important question is why the energetic stabilities of HG bps appear to be only weakly dependent on sequence. Further studies are required to understand the structure and specific interactions that may help stabilize the TS and HG bp.
Methods
NMR samples and resonance assignments
Unlabelled DNA samples were purchased as single-stranded oligos from Integrated DNA Technologies (IDT) with standard desalting purification. The DNA oligos were resuspended to ~200 μM in 15 mM phosphate buffer with corresponding pH (see below), 25 mM NaCl, 0.1 mM EDTA. Duplexes were annealed by mixing an equimolar ratio of the complementary DNA strands, heating at 95 °C for 2 min followed by gradual cooling at room temperature for ~30 min. Unlabelled DNA duplexes were washed 3 × in resuspension buffer by microcentrifugation using an Amicon Ultra-4 centrifugal filter with a 3-kDa cutoff, concentrated to ~2–3 mM and ~250 μl, then supplied with 10% D2O. Natural abundance CG3 was resuspended in~4 ml of milliQ H2O and dialyzed against 2 l of milliQ H2O with two exchanges for a total of 6 l of milliQ H2O, using a dialysis tube from G-Biosciences with a 1-kDa cutoff. Dialyzed CG3 was lyophilized and resuspended in NMR buffer to ~4 mM and supplied with 10% D2O. Hemi-13C/15N-labelled A5 duplex was prepared by annealing a uniformly 13C/15N-labelled thymine-rich strand to a natural abundance adenosine-rich strand. Fully 13C/15N-labelled DNA duplexes were prepared by annealing two labelled strands together. All labelled single strands were synthesized in vitro by the method of Zimmer and Crothers41 using a DNA hairpin template with a 5′ overhang corresponding to the complement of the target labelled strand and a 3′ ribose (IDT). In this study, we used the same hairpin sequence as Zimmer and Crothers41, Klenow fragment DNA polymerase (NEB) NEB2 buffer (NEB) and uniformly 13C/15N-labelled dNTPs (Isotec, Sigma-Aldrich and Silantes). Base- and heat-catalyzed cleavage separated the hairpin template from the 13C/15N-labelled synthesized product. The single-stranded DNA product was purified by 20% denaturing polyacrylamide gel electrophoresis, isolated by passive elution from crushed gel pieces and desalted on a C18 reverse-phase column (Sep-Pak, Waters). The oligo was lyophilized and suspended in NMR buffer. The semi-labelled DNA samples were prepared by titrating the unlabelled strand directly into an NMR tube containing the 13C/15N-labelled strand and monitoring the disappearance of single-stranded DNA peaks using heteronuclear single quantum coherence (HSQC) experiments. The fully labelled samples were annealed in a similar fashion. 2D 1H–1H nuclear overhauser spectroscopy (NOESY) experiments at 26 °C (A2, A4, A5, A6, CA3, E) or 25 °C (CG3 and Z junction (ZJXN)) and pH 5.2 (A5), 5.4 (A2, A4, A6, CA3, CG3), 6.8 (E) or 7.5 (ZJXN) were used to assign resonances as described previously1. See NMR R1ρ relaxation dispersion for R1ρ RD pH values.
NMR R1ρ RD
All NMR experiments were performed on a Bruker Avance 600 MHz NMR spectrometer equipped with a 5-mm triple-resonance cryogenic probe. R1ρ RD experiments were performed at pH 5.2–7.5 and 5.2–5.4 for A·T and G·C+ bps, respectively. Buffer conditions for R1ρ RD measurements are 15 mM sodium phosphate, 25 mM NaCl, 0.1 mM EDTA, 10% D2O pH=5.2 (A5, A4: C15 C6, A16 C1′, G10 C1′), pH=5.4 (A2, A4, A6, CA3, CG3), pH=6.8 (E) and pH=7.5 (ZJXN) at 26 °C (A2, A4, A5, A6, CA3, E) or 25 °C (A2 A3 C1′, CG3, ZJXN). CG3 and E are unlabelled DNA samples, the T-rich strand of A5 is 13C/15N labelled while A6, A4, A2, CA3 and ZJXN are fully 13C/15N-labelled. We use low-pH conditions for transient (and protonated) G·C+ bps in order to increase the WC-to-HG chemical exchange signature detected by R1ρ RD3. We recently reported a detailed analysis of the pH dependence of chemical exchange corresponding to transient HG bp formation and how measurements at such lower pH conditions can be qualitatively interpolated to assess exchange at higher pH3. Carbon and nitrogen R1ρ RD profiles for guanine/adenine C8, guanine/adenine C1′, cytosine C6, guanine N1 and thymine N3 were measured using a one-dimensional acquisition scheme which uses selective Hartmann–Hahn polarization transfer42 to selectively excite one C–H or N–H spin system at a time6. The spin lock power and offset frequencies are summarized in Supplementary Table 4. The following delays were used: A2: A3 C1′, G10 C1′, A17 C1′; A4: A5 C1′, G10 C1′, A16 C1′, A19 C1′; CA3: G10 C1′; A5: A3 C1′, C5 C1′ (0, 4, 8, 12, 18, 26, 34, 42, 12, 42); A5 G11 C8 (0, 12, 32, 26, 32); A4 A17 C8, A6 A17 C8 (0, 4, 12, 32, 26, 32); A2: C2 C6, T9 C6, G11 C8, A16 C8, A17 C2; CA3: A16 C8, C17 C6, C19 C6, A21 C1′; A4: C15 C6, G11 C8; A5: C9 C6, G10 C8; A6: G10 C8, A16 C8 (0, 4, 8, 12, 16, 20, 26, 32, 12, 32); A2: T8 N3, G10 N1, G23 N1; A5: T4 N3, T5 N3, T6 N3, T7 N3, T8 N3; A6 G10 N1 (0, 8, 16, 24, 36, 48, 60, 80, 100, 16, 70, 100); CG3 G4 C8 (0, 60, 60); ZJXN: A6 C8, A24 C8 (0, 4, 8, 12, 16, 20, 24, 30, 12, 30); E A5 C8: (0, 48, 48). Data points meeting C-C Hartmann–Hahn matching conditions were omitted as described previously6. Data were processed using NMRPipe43 and R1ρ values were determined from monoexponential decay fits of the resonance intensities using a script44 in Mathematica 9 (Wolfram Research). On- and off-resonance R1ρ data were fit to the Laguerre equation45 (equation 2) using Origin 8.6 (OriginLab),

Where  ,
,  and
and  . ΔωAB=ΩB−ΩA, where ΩA and ΩB are the chemical shifts of the ground (A) and transient (B) states, respectively, in Hz. R1 and R2 are the intrinsic longitudinal and transverse relaxation rate constants, respectively, and are assumed to be identical for the ground (A) and transient (B) states. θ=arctan(ω1/Ω) where ω1 is the spin lock power strength. Ω=Ωobs−ωrf where Ω is the offset of the spin lock carrier frequency (ωrf) from the observed resonance frequency (Ωobs). Ωobs=pAΩA+pBΩB where pA and pB are the ground and transient state populations, respectively, and pA+pB=1. kex is the chemical exchange rate constant for a two-state exchange process where kex=kA+kB, kA=kexpB and kB=kexpA. kA and kB are the forward and reverse rate constants, respectively.
. ΔωAB=ΩB−ΩA, where ΩA and ΩB are the chemical shifts of the ground (A) and transient (B) states, respectively, in Hz. R1 and R2 are the intrinsic longitudinal and transverse relaxation rate constants, respectively, and are assumed to be identical for the ground (A) and transient (B) states. θ=arctan(ω1/Ω) where ω1 is the spin lock power strength. Ω=Ωobs−ωrf where Ω is the offset of the spin lock carrier frequency (ωrf) from the observed resonance frequency (Ωobs). Ωobs=pAΩA+pBΩB where pA and pB are the ground and transient state populations, respectively, and pA+pB=1. kex is the chemical exchange rate constant for a two-state exchange process where kex=kA+kB, kA=kexpB and kB=kexpA. kA and kB are the forward and reverse rate constants, respectively.
Plots of R1ρ data are presented as R2eff (R2eff=R2+Rex) in Fig. 1c and Supplementary Fig. 1 where,

In cases where large errors were accompanied by small Rex, model selection comparing presence and absence of exchange was carried out using the F- and Akaike’s Information Criterion (AIC)46 tests to discriminate between exchange and no detectable exchange (data not shown). The F-test compares two nested models fit to the same data under the null hypothesis that the residual sum of squares of the less complex, restricted model is not significantly larger than that of the more complex, full model. The AIC test assesses the likelihood of a model given the data and seeks to minimize loss of information embedded within the data. For A2 G23 N1, no exchange is the selected model under the conditions used. Because of lower sensitivity to exchange, we did not interpret absence of 15N dispersion as evidence for absence of transient HG bps.
The free-energy difference between the WC GS and HG transient state (ΔGWC−HG) was computed using,

where k1 and k2 are the forward and reverse rate constants, respectively, h is Planck’s constant, kB is Boltzmann’s constant, R is the gas constant and T is temperature. The forward barrier of the transition (ΔG‡WC−HG) was computed using,

where κ is the transmission coefficient which is assumed to be unity.
CD melting
DNA duplexes (IDT) were prepared using ultracentrifugation as described in NMR Samples and Resonance Assignments in 15 mM phosphate buffer, 0.1 mM EDTA, 25 mM NaCl pH 5.4 and supplied with 10% D2O. Duplexes were prepared by diluting complementary single-stranded stocks to 50 μM in the same tube with a final volume of 200 μl. Samples were denatured at 95 °C for 5 min followed by annealing of at least 10 min on the bench top. Samples were transferred to a 1-mm cuvette (Starna Cells), mineral oil was added to the top of the solution and the cuvette was capped. Melting experiments were performed on a Jasco Spectropolarimeter equipped with a recirculating water bath and Peltier temperature control unit. Temperature ramps were performed from 5 to 80 °C with a bandwidth of 5 nm (1 nm for ZJXN), ramp rate of 1 °C min−1, equilibration time of 20 s and sensitivity of 100 millidegrees. Wavelength scans used the same sensitivity and bandwidth as melting runs. Spectral measurements were performed between 220 to 330 nm with a scan rate of 100 nm min−1. Temperature ramp profiles were fit to the Boltzmann model47, which has previously been used to determine nucleic acid melting temperatures48,

where θ is the elipticity at 254 nm normalized to the signal change magnitude, LL and UL are the lower and upper limits of the transition, respectively, a is the Hill slope, Tm is the melting temperature defined as the point of inflection of the melting curve and T is the independent variable temperature.
Phi (Φ)-value analysis
Φ-value analysis34 was carried out by computing Φ using equation 1 (Φ=ΔΔGTS−WC/ΔΔGWC−HG) where ΔΔGTS−WC=ΔG‡WC−HG,mut−ΔG‡WC−HG,Ψ−WT and ΔΔGWC−HG=ΔGWC–HG,mut−ΔGWC−HG,Ψ−WT are the change in the forward free-energy barrier (ΔG‡WC−HG) and free-energy difference between WC and HG bps (ΔGWC−HG), respectively, on introduction of one or multiple mutations (mut) as compared with ‘wild-type’ (Ψ−WT). We defined Ψ-WT to be A5 T4 N3 and A2 G10 N1 for A·T and G·C+ bps, respectively, given that they have the lowest ΔGWC−HG values. To control for systematic errors in Φ arising due to use of a 15N Ψ−WT reference resonance, we also performed Φ-value analysis assigning a 13C resonance as Ψ−WT for A·T and G·C+ bps, respectively. In all cases we observe Φ-values concentrated around ~1 consistent with a ‘late’ TS. Note that Φ≈ 0 when ΔΔGTS−WC≈0 relative to ΔΔGWC−HG implying an early WC-like TS, whereas Φ≈1 when ΔΔGTS−WC≈ΔΔGWC−HG and implies a late HG-like TS. Errors for calculated Φ-values for A6 A17 C8, A2 A3 C1′, A4 C15 C6 and A5 G10 C8 were larger than the corresponding values and thus could not be determined accurately.
Additional information
How to cite this article: Alvey, H. S. et al. Widespread transient Hoogsteen base pairs in canonical duplex DNA with variable energetics. Nat. Commun. 5:4786 doi: 10.1038/ncomms5786 (2014).
References
Nikolova, E. N. et al. Transient Hoogsteen base pairs in canonical duplex DNA. Nature 470, 498–502 (2011).
Nikolova, E. N., Gottardo, F. L. & Al-Hashimi, H. M. Probing transient Hoogsteen hydrogen bonds in canonical duplex DNA using NMR relaxation dispersion and single-atom substitution. J. Am. Chem. Soc. 134, 3667–3670 (2012).
Nikolova, E. N., Goh, G. B., Brooks, C. L. 3rd & Al-Hashimi, H. M. Characterizing the protonation state of cytosine in transient G.C Hoogsteen base pairs in duplex DNA. J. Am. Chem. Soc. 135, 6766–6769 (2013).
Palmer, A. G. 3rd Chemical exchange in biomacromolecules: past, present, and future. J. Magn. Reson. 241, 3–17 (2014).
Sekhar, A. & Kay, L. E. NMR paves the way for atomic level descriptions of sparsely populated, transiently formed biomolecular conformers. Proc. Natl Acad. Sci. USA 110, 12867–12874 (2013).
Hansen, A. L., Nikolova, E. N., Casiano-Negroni, A. & Al-Hashimi, H. M. Extending the range of microsecond-to-millisecond chemical exchange detected in labeled and unlabeled nucleic acids by selective carbon R(1rho) NMR spectroscopy. J. Am. Chem. Soc. 131, 3818–3819 (2009).
Hoogsteen, K. The structure of crystals containing a hydrogen-bonded complex of 1-methylthymine and 9-methyladenine. Acta Crystallogr. 12, 822–823 (1959).
Nikolova, E. N. et al. A historical account of hoogsteen base-pairs in duplex DNA. Biopolymers 99, 955–968 (2013).
Rice, P. A., Yang, S., Mizuuchi, K. & Nash, H. A. Crystal structure of an IHF-DNA complex: a protein-induced DNA U-turn. Cell 87, 1295–1306 (1996).
Aishima, J. et al. A Hoogsteen base pair embedded in undistorted B-DNA. Nucleic Acids Res. 30, 5244–5252 (2002).
Patikoglou, G. A. et al. TATA element recognition by the TATA box-binding protein has been conserved throughout evolution. Genes Dev. 13, 3217–3230 (1999).
Kitayner, M. et al. Diversity in DNA recognition by p53 revealed by crystal structures with Hoogsteen base pairs. Nat. Struct. Mol. Biol. 17, 423–429 (2010).
Bohnuud, T. et al. Computational mapping reveals dramatic effect of Hoogsteen breathing on duplex DNA reactivity with formaldehyde. Nucleic Acids Res. 40, 7644–7652 (2012).
Yang, W. Structure and mechanism for DNA lesion recognition. Cell Res. 18, 184–197 (2008).
Yang, H., Zhan, Y., Fenn, D., Chi, L. M. & Lam, S. L. Effect of 1-methyladenine on double-helical DNA structures. FEBS Lett. 582, 1629–1633 (2008).
Nair, D. T., Johnson, R. E., Prakash, S., Prakash, L. & Aggarwal, A. K. Replication by human DNA polymerase-iota occurs by Hoogsteen base-pairing. Nature 430, 377–380 (2004).
Makarova, A. V. & Kulbachinskiy, A. V. Structure of human DNA polymerase iota and the mechanism of DNA synthesis. Biochemistry (Moscow) 77, 547–561 (2012).
Harris, R. C. et al. Opposites attract: shape and electrostatic complementarity in protein-DNA complexes. inInnovations in Biomolecular Modeling and Simulations Vol 2, ed. Schlick T. 53–80Royal Soc Chemistry (2012).
Rohs, R. et al. The role of DNA shape in protein-DNA recognition. Nature 461, 1248–1253 (2009).
Johnson, R. E., Prakash, L. & Prakash, S. Biochemical evidence for the requirement of Hoogsteen base pairing for replication by human DNA polymerase iota. Proc. Natl Acad. Sci. USA 102, 10466–10471 (2005).
Liu, K., Miles, H. T., Frazier, J. & Sasisekharan, V. A novel DNA duplex. A parallel-stranded DNA helix with Hoogsteen base pairing. Biochemistry 32, 11802–11809 (1993).
Abrescia, N. G., Gonzalez, C., Gouyette, C. & Subirana, J. A. X-ray and NMR studies of the DNA oligomer d(ATATAT): Hoogsteen base pairing in duplex DNA. Biochemistry 43, 4092–4100 (2004).
Abrescia, N. G., Thompson, A., Huynh-Dinh, T. & Subirana, J. A. Crystal structure of an antiparallel DNA fragment with Hoogsteen base pairing. Proc. Natl Acad. Sci. USA 99, 2806–2811 (2002).
Cubero, E., Luque, F. J. & Orozco, M. Theoretical study of the Hoogsteen-Watson-Crick junctions in DNA. Biophys. J. 90, 1000–1008 (2006).
Cubero, E., Abrescia, N. G., Subirana, J. A., Luque, F. J. & Orozco, M. Theoretical study of a new DNA structure: the antiparallel Hoogsteen duplex. J. Am. Chem. Soc. 125, 14603–14612 (2003).
Ughetto, G. et al. A comparison of the structure of echinomycin and triostin A complexed to a DNA fragment. Nucleic Acids Res. 13, 2305–2323 (1985).
Gilbert, D. E., van der Marel, G. A., van Boom, J. H. & Feigon, J. Unstable Hoogsteen base pairs adjacent to echinomycin binding sites within a DNA duplex. Proc. Natl Acad. Sci. USA 86, 3006–3010 (1989).
Schwartz, T., Rould, M. A., Lowenhaupt, K., Herbert, A. & Rich, A. Crystal structure of the Zalpha domain of the human editing enzyme ADAR1 bound to left-handed Z-DNA. Science 284, 1841–1845 (1999).
Bothe, J. R., Lowenhaupt, K. & Al-Hashimi, H. M. Sequence-specific B-DNA flexibility modulates Z-DNA formation. J. Am. Chem. Soc. 133, 2016–2018 (2011).
Ha, S. C., Lowenhaupt, K., Rich, A., Kim, Y. G. & Kim, K. K. Crystal structure of a junction between B-DNA and Z-DNA reveals two extruded bases. Nature 437, 1183–1186 (2005).
SantaLucia, J. Jr, Allawi, H. T. & Seneviratne, P. A. Improved nearest-neighbor parameters for predicting DNA duplex stability. Biochemistry 35, 3555–3562 (1996).
Coman, D. & Russu, I. M. A nuclear magnetic resonance investigation of the energetics of basepair opening pathways in DNA. Biophys. J. 89, 3285–3292 (2005).
Gueron, M., Kochoyan, M. & Leroy, J. L. A single mode of DNA base-pair opening drives imino proton exchange. Nature 328, 89–92 (1987).
Fersht, A. R., Matouschek, A. & Serrano, L. The folding of an enzyme. I. Theory of protein engineering analysis of stability and pathway of protein folding. J. Mol. Biol. 224, 771–782 (1992).
Neudecker, P., Zarrine-Afsar, A., Davidson, A. R. & Kay, L. E. Phi-value analysis of a three-state protein folding pathway by NMR relaxation dispersion spectroscopy. Proc. Natl Acad. Sci. USA 104, 15717–15722 (2007).
Wang, J. DNA polymerases: Hoogsteen base-pairing in DNA replication? Nature 437, E6–E7 discussion E7 (2005).
Honig, B. & Rohs, R. Biophysics: flipping Watson and Crick. Nature 470, 472–473 (2011).
Ronning, D. R. et al. Active site sharing and subterminal hairpin recognition in a new class of DNA transposases. Mol. Cell 20, 143–154 (2005).
Wang, A. H. et al. The molecular structure of a DNA-triostin A complex. Science 225, 1115–1121 (1984).
Zhang, Y., Xi, Z., Hegde, R. S., Shakked, Z. & Crothers, D. M. Predicting indirect readout effects in protein-DNA interactions. Proc. Natl Acad. Sci. USA 101, 8337–8341 (2004).
Zimmer, D. P. & Crothers, D. M. NMR of enzymatically synthesized uniformly 13C15N-labeled DNA oligonucleotides. Proc. Natl Acad. Sci. USA 92, 3091–3095 (1995).
Pelupessy, P., Chiarparin, E. & Bodenhausen, G. Excitation of selected proton signals in NMR of isotopically labeled macromolecules. J. Magn. Reson. 138, 178–181 (1999).
Delaglio, F. et al. Nmrpipe–a multidimensional spectral processing system based on unix pipes. J. Biomol. NMR 6, 277–293 (1995).
Spyracopoulos, L. A suite of mathematica notebooks for the analysis of protein main chain 15N NMR relaxation data. J. Biomol. NMR 36, 215–224 (2006).
Miloushev, V. Z. & Palmer, A. G. 3rd R(1rho) relaxation for two-site chemical exchange: general approximations and some exact solutions. J. Magn. Reson. 177, 221–227 (2005).
Akaike, H. A new look at the statistical model identification. IEEE Trans. Aut. Contr. 19, 716–723 (1974).
Schulz, M. N., Landstrom, J. & Hubbard, R. E. MTSA-a Matlab program to fit thermal shift data. Anal. Biochem. 433, 43–47 (2013).
Doktycz, M. J., Morris, M. D., Dormady, S. J., Beattie, K. L. & Jacobson, K. B. Optical melting of 128 octamer DNA duplexes: effects of base pair location and nearest neighbors on thermal stability. J. Biol. Chem. 270, 8439–8445 (1995).
Acknowledgements
We thank Dr Vivekanandan Subramanian for maintenance of the NMR instrument. We gratefully acknowledge Professor Ari Gafni for access to the CD instrument and Dr Joseph Schauerte for maintenance of the CD instrument. This work was supported by NIH grant GM089846 awarded to H.M.A.-H.
Author information
Authors and Affiliations
Contributions
H.S.A., F.L.G. and H.M.A.-H. conceived the idea; H.S.A., F.L.G. and E.N.N. prepared samples and measured NMR data; H.S.A. carried out the data analysis with help from F.L.G., E.N.N. and H.M.A.-H.. H.S.A. and H.M.A.-H. wrote the manuscript with help from F.L.G. and E.N.N.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-4 and Supplementary Tables 1-4 (PDF 2181 kb)
Rights and permissions
About this article
Cite this article
Alvey, H., Gottardo, F., Nikolova, E. et al. Widespread transient Hoogsteen base pairs in canonical duplex DNA with variable energetics. Nat Commun 5, 4786 (2014). https://doi.org/10.1038/ncomms5786
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms5786
This article is cited by
-
Dynamic basis for dA•dGTP and dA•d8OGTP misincorporation via Hoogsteen base pairs
Nature Chemical Biology (2023)
-
Slow motions in A·T rich DNA sequence
Scientific Reports (2020)
-
DNA mismatches reveal conformational penalties in protein–DNA recognition
Nature (2020)
-
Alternated Branching Ratios by Anomaly in Collision-Induced Dissociation of Proton-Bound Hoogsteen Base Pairs of 1-Methylcytosine with 1-Methylguanine and 9-Methylguanine
Journal of the American Society for Mass Spectrometry (2019)
-
Non-dissociative structural transitions of the Watson-Crick and reverse Watson-Crick А·Т DNA base pairs into the Hoogsteen and reverse Hoogsteen forms
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.