Abstract
Vapour–liquid–solid route and its variants are routinely used for scalable synthesis of semiconducting nanowires, yet the fundamental growth processes remain unknown. Here we employ atomic-scale computations based on model potentials to study the stability and growth of gold-catalysed silicon nanowires. Equilibrium studies uncover segregation at the solid-like surface of the catalyst particle, a liquid AuSi droplet, and a silicon-rich droplet–nanowire interface enveloped by heterogeneous truncating facets. Supersaturation of the droplets leads to rapid one-dimensional growth on the truncating facets and much slower nucleation-controlled two-dimensional growth on the main facet. Surface diffusion is suppressed and the excess Si flux occurs through the droplet bulk which, together with the Si-rich interface and contact line, lowers the nucleation barrier on the main facet. The ensuing step flow is modified by Au diffusion away from the step edges. Our study highlights key interfacial characteristics for morphological and compositional control of semiconducting nanowire arrays.
Similar content being viewed by others
Introduction
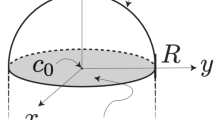
A characteristic feature of the vapour–liquid–solid (VLS) route for nanowire synthesis is the presence of a catalyst particle, usually a droplet, that mediates the mass transfer from the vapour phase precursor to the growing nanowire2,3,4,5,6,7. The technique was demonstrated successfully in the 1960s in the context of crystalline whisker growth2, and has become increasingly relevant of late as it offers direct control over nanowire composition and morphology, aspects of critical importance in several nanowire-based applications8,9,10,11,12,13,14,15,16,17,18,19. The salient features can be readily seen in the schematic illustration in Fig. 1a (inset). A low melting eutectic droplet is supersaturated following breakdown of the precursor gas on its surface. Past a critical point, crystallization at the droplet–nanowire interface results in tip growth. The droplet then acts as a conduit for one-dimensional (1D) crystal growth with diameters that are set by its dimensions.
(a) Bulk phase diagram for the Au–Si binary system predicted by the model inter-atomic interactions employed in this study (solid black line) and the size correction to the model diagram  (solid red circle) based on doubly capped SiNW computations shown in b. The solid grey line is the experimental bulk phase diagram shown for comparison (inset). Schematic illustrating the VLS route for SiNW nanowire synthesis. (b, left) Atomic configuration of an equilibrated 10-nm-long SiNW capped with catalyst particles at both ends. (right) Mid-section slice through the droplet–nanowire system that reveals the shape of the droplets and the faceted nature of the solid nanowire. Pale yellow is solid silicon, and red and bright yellow indicate liquid Si and Au, respectively. For consistency, the colour scheme for the atomic plots and the density profiles is preserved in the remaining figures.
(solid red circle) based on doubly capped SiNW computations shown in b. The solid grey line is the experimental bulk phase diagram shown for comparison (inset). Schematic illustrating the VLS route for SiNW nanowire synthesis. (b, left) Atomic configuration of an equilibrated 10-nm-long SiNW capped with catalyst particles at both ends. (right) Mid-section slice through the droplet–nanowire system that reveals the shape of the droplets and the faceted nature of the solid nanowire. Pale yellow is solid silicon, and red and bright yellow indicate liquid Si and Au, respectively. For consistency, the colour scheme for the atomic plots and the density profiles is preserved in the remaining figures.
The atomic-scale structure, energetics and dynamics of the alloyed particle directly influence the morphology and composition of the as-grown nanowires, yet the prevailing ideas continue to be based on continuum frameworks13,15,20,21,22,23,24,25. As an example, gold-catalysed growth of silicon nanowires is a well-studied system yet the morphology and composition of the particle–nanowire interface is unknown. Basic crystal growth parameters such as the size-dependent equilibrium composition  above which the droplet rejects the excess silicon onto the nanowire remain unknown; continuum models ignore the effect of segregation and related interfacial phenomena. Such microscopic features modify the droplet morphology through the balance of interfacial tension at the contact line, which in turn dynamically regulates the chemical potential difference between the two phases, or the driving force for growth. Likewise, the atomic-scale processes that sustain the growth of the nanowire remain unknown. The steady state is limited by reduction of the precursor gas on the droplet surface26 and the two-dimensional (2D) nucleation-limited step flow at the droplet–nanowire interface27,27. Understanding the interplay with the dynamics that feeds the growth of the nuclei is crucial for compositional control of the as-grown nanowire17,29,30,31 and requires an atomistic understanding of the near-equilibrium particle behaviour.
above which the droplet rejects the excess silicon onto the nanowire remain unknown; continuum models ignore the effect of segregation and related interfacial phenomena. Such microscopic features modify the droplet morphology through the balance of interfacial tension at the contact line, which in turn dynamically regulates the chemical potential difference between the two phases, or the driving force for growth. Likewise, the atomic-scale processes that sustain the growth of the nanowire remain unknown. The steady state is limited by reduction of the precursor gas on the droplet surface26 and the two-dimensional (2D) nucleation-limited step flow at the droplet–nanowire interface27,27. Understanding the interplay with the dynamics that feeds the growth of the nuclei is crucial for compositional control of the as-grown nanowire17,29,30,31 and requires an atomistic understanding of the near-equilibrium particle behaviour.
We focus exclusively on a small diameter 2R=10 nm,  111
111 silicon nanowire growing isothermally above the eutectic temperature at T=873 K. Both experiments and ab-initio computational techniques are handicapped in accessing the spatio-temporal scales associated with the energetics and three-dimensional (3D) dynamics during growth32,33,34. We capture these processes in their full complexity by employing atomistic simulations based on an angular embedded-atom-method (AEAM) framework for describing the hybrid metallic–covalent interactions35. The computed surfaces of equilibrated isolated droplets are ordered and Si rich, yet decorated by a submonolayer of Au. The surface structure leads to sluggish atomic mobilities such that most of the excess Si flux during growth is directed through the droplet bulk. Studies on droplet-capped SiNWs show that the solid–liquid interface is again Si rich and the nanowire sidewalls are decorated by Au monolayers. Of note is the interface morphology composed of a main facet that truncates into smaller staircase-like, stepped facets at the contact line. The latter grow rapidly in a 1D manner while crystallization on the main facet proceeds via nucleation of 2D islands, which then grow via step flow. Interestingly, the motion of the steps is limited by Au diffusion into the droplets and/or the nanowire sidewalls. Studies with varying levels of droplet supersaturation yield the nucleation barrier, which is lower than that for homogeneous nucleation on a planar Si(111)–AuSi interface, highlighting the combined role of segregation, atomic diffusivities and interface morphology in regulating nanowire growth.
silicon nanowire growing isothermally above the eutectic temperature at T=873 K. Both experiments and ab-initio computational techniques are handicapped in accessing the spatio-temporal scales associated with the energetics and three-dimensional (3D) dynamics during growth32,33,34. We capture these processes in their full complexity by employing atomistic simulations based on an angular embedded-atom-method (AEAM) framework for describing the hybrid metallic–covalent interactions35. The computed surfaces of equilibrated isolated droplets are ordered and Si rich, yet decorated by a submonolayer of Au. The surface structure leads to sluggish atomic mobilities such that most of the excess Si flux during growth is directed through the droplet bulk. Studies on droplet-capped SiNWs show that the solid–liquid interface is again Si rich and the nanowire sidewalls are decorated by Au monolayers. Of note is the interface morphology composed of a main facet that truncates into smaller staircase-like, stepped facets at the contact line. The latter grow rapidly in a 1D manner while crystallization on the main facet proceeds via nucleation of 2D islands, which then grow via step flow. Interestingly, the motion of the steps is limited by Au diffusion into the droplets and/or the nanowire sidewalls. Studies with varying levels of droplet supersaturation yield the nucleation barrier, which is lower than that for homogeneous nucleation on a planar Si(111)–AuSi interface, highlighting the combined role of segregation, atomic diffusivities and interface morphology in regulating nanowire growth.
Results
Size-corrected liquidus composition
We first quantify the equilibrium state of the alloy system by extracting the liquidus composition,  (2R, T=873 K). We capture the effect of the solid–liquid interface and the contact line by extracting the equilibrium state of a 2R=10 nm diameter,
(2R, T=873 K). We capture the effect of the solid–liquid interface and the contact line by extracting the equilibrium state of a 2R=10 nm diameter,  111
111 SiNW capped by a droplet at each end. The geometry allows the nanowire to act as a source/sink as the droplets adjust to the size- and morphology-dependent liquidus composition. The starting configuration consists of a nanowire with a hexagonal cross-section bounded by {112} -like sidewall facets (Methods). In particular, we have performed separate computations for SiNWs with {110} and {112} sidewall facets. The choices are motivated by past experiments that have shown that the sidewalls can be a mixture of {112} and {110} facets14,36. Figure 1b shows the atomic configuration of the equilibrated SiNW system, in this particular case bounded by {110} facets. We immediately see the formation of an Au submonolayer on the sidewalls, consistent with prior experimental observations37. The cross-section maintains its sixfold symmetry. Similar behavior is observed in simulations of SiNW with {112} family of facets, implying that the two surfaces are energetically quite close; we do not observe their co-existence14,36. This is not surprising as the thermodynamic shape is inherently size dependent38 and the diameters of the synthesized SiNWs are much larger. The {112} facet is unstable for Au coverages typically observed during nanowire growth14, yet we do not observe axial serrations due to the small nanowire lengths. We also cannot rule out the effects of surface stresses at these small sizes39, as they can change the relative stability between {110} and {112} family of facets. The average composition within the interior of the capping droplets is
SiNW capped by a droplet at each end. The geometry allows the nanowire to act as a source/sink as the droplets adjust to the size- and morphology-dependent liquidus composition. The starting configuration consists of a nanowire with a hexagonal cross-section bounded by {112} -like sidewall facets (Methods). In particular, we have performed separate computations for SiNWs with {110} and {112} sidewall facets. The choices are motivated by past experiments that have shown that the sidewalls can be a mixture of {112} and {110} facets14,36. Figure 1b shows the atomic configuration of the equilibrated SiNW system, in this particular case bounded by {110} facets. We immediately see the formation of an Au submonolayer on the sidewalls, consistent with prior experimental observations37. The cross-section maintains its sixfold symmetry. Similar behavior is observed in simulations of SiNW with {112} family of facets, implying that the two surfaces are energetically quite close; we do not observe their co-existence14,36. This is not surprising as the thermodynamic shape is inherently size dependent38 and the diameters of the synthesized SiNWs are much larger. The {112} facet is unstable for Au coverages typically observed during nanowire growth14, yet we do not observe axial serrations due to the small nanowire lengths. We also cannot rule out the effects of surface stresses at these small sizes39, as they can change the relative stability between {110} and {112} family of facets. The average composition within the interior of the capping droplets is  (2R)=0.46 and is indicated on the phase diagram in Fig. 1a. The size-corrected supersaturation with respect to the bulk liquidus composition is
(2R)=0.46 and is indicated on the phase diagram in Fig. 1a. The size-corrected supersaturation with respect to the bulk liquidus composition is  and the equivalent undercooling ΔT≈18 K.
and the equivalent undercooling ΔT≈18 K.
Surface segregation, ordering and kinetics
The composition of the capping droplets is non-uniform and we decouple the surface effects by first studying isolated droplets at  . Atomic configuration of a midsection through an equilibrated droplet is shown in Fig. 2a. The non-uniform composition arises mainly due to surface segregation. Details are extracted using normalized density profiles
. Atomic configuration of a midsection through an equilibrated droplet is shown in Fig. 2a. The non-uniform composition arises mainly due to surface segregation. Details are extracted using normalized density profiles  plotted as a function of the distance r from the droplet centre of mass (Fig. 2b). The overall profile (dark solid line) has a well-defined peak at the surface suggestive of a solid-like surface structure. The multiple alternating peaks in the Au and Si profiles indicate a Si-rich subsurface decorated by a submonolayer of Au. Although the Si segregation is consistent with experiments on eutectic AuSi thin films40, the Au submonolayer has not been reported earlier.
plotted as a function of the distance r from the droplet centre of mass (Fig. 2b). The overall profile (dark solid line) has a well-defined peak at the surface suggestive of a solid-like surface structure. The multiple alternating peaks in the Au and Si profiles indicate a Si-rich subsurface decorated by a submonolayer of Au. Although the Si segregation is consistent with experiments on eutectic AuSi thin films40, the Au submonolayer has not been reported earlier.
(a) Midsection through an atomic plot of a 32,000-atom AuSi system equilibrated at the model liquidus composition  . Radial variation of (b) the ensemble averaged and normalized density profile
. Radial variation of (b) the ensemble averaged and normalized density profile  for the combined system (solid dark line) and for Au and Si, as indicated, and (c) ensemble averaged diffusivities of Au and Si, DAu/Si(r), extracted from equilibrium MD simulations of the droplets. The dotted lines in c correspond to the bulk diffusivities extracted from simulations on an equilibrated bulk alloy system (see Methods).
for the combined system (solid dark line) and for Au and Si, as indicated, and (c) ensemble averaged diffusivities of Au and Si, DAu/Si(r), extracted from equilibrium MD simulations of the droplets. The dotted lines in c correspond to the bulk diffusivities extracted from simulations on an equilibrated bulk alloy system (see Methods).
We further isolate the effects of surface curvature via segregation studies on thin AuSi films and the results are summarized in Supplementary Fig. S1. At the eutectic point, XSi≈0.31 and T=590 K, we see ripples in the density profiles that extend to ~7 layers and decay rapidly into the bulk. The density variations are larger than those usually seen on liquid surfaces and suggest a compositionally ordered pre-frozen surface qualitatively similar to the surface ordering seen in eutectic AuSi thin films40. The ordering decreases with increasing XSi and temperature, and the extracted density profiles are similar to those observed in the liquid droplets, that is, the surface curvature has little effect. The surfaces are always decorated by a Au submonolayer. It is expectedly less ordered compared with monolayers on crystalline Si surfaces, and this feature likely makes it difficult to be resolved by X-ray-based surface characterization techniques40. The discrepancy can certainly be an artifact of the EAM framework used to describe the metallic bonds41; it underestimates the surface tension of liquid Au by 20% and can therefore artificially favour the formation of the Au submonolayer. Nonetheless, it is natural to expect that the stability of Au monolayers on crystalline Si surfaces42 also extends to partially crystalline Si surfaces. As confirmation, we still see the Au decoration in segregations studies with modified Au–Au interactions using a charge gradient approach fit to the surface tension of liquid Au41 (Supplementary Fig. S2 and Supplementary Methods). The Au coverage is slightly reduced in extent yet the qualitative trends remain unchanged. The effect of surface structure extends to the diffusive kinetics as well. We quantify the interplay by monitoring the atomic mean-square displacements (MSDs) within equilibrium molecular dynamics (MD) simulations of the droplets (Methods) that yield the atomic diffusivities DAu/Si (Supplementary Fig. S3). The radial variation is plotted in Fig. 2c. For both Au and Si, the surface diffusivity is less than half of the bulk value and further highlights the dramatic effect of the surface structure.
Equilibrated nanowire–particle system
The substrate is explicitly included by mating an equilibrated droplet–SiNW system onto a dehydrogenated Si(111) surface (Methods). Figure 3a and Supplementary Fig. S4 show the atomic configurations of the nanowire system with {110} and {112} sidewall facets (vertical), respectively. A stable Au submonolayer is evident on the nanowire sidewalls (right, Fig. 3a). The surface segregation is similar to that on isolated droplets (Fig. 2b), that is, the substrate has a minor effect. There are differences in the structure of the Au submonolayer for the two classes of sidewall facets. We see Si segregation at the droplet–nanowire interface within liquid layers adjacent to the interface and parallel to (111) planes. Ripples in the in-plane density profiles within the droplet extracted as a function of distance from the interface z extend to a thickness of ≈5Å (Fig. 3b). The sharp Si and Au peaks adjacent to the interface at z≈1.5Å (arrow) indicate a Si-rich layer with some in-plane order. The remainder of the segregated layer is mostly liquid characterized by a smaller and broader Si peak at z≈2.5Å (arrow), followed by a Au-rich region (z≈4Å), which ultimately settles into the bulk composition. The compositional distribution for the entire nanowire–droplet system is summarized schematically in Fig. 3c.
(a, left) Equilibrium atomic configuration of a 2R=10 nm diameter  111
111 silicon nanowire with {110} sidewall facets. The wire is grown on a (111) silicon substrate and is capped by an AuSi catalyst particle at the liquidus composition,
silicon nanowire with {110} sidewall facets. The wire is grown on a (111) silicon substrate and is capped by an AuSi catalyst particle at the liquidus composition,  . (a, middle, inset) The surface plot viewed along the [111] direction showing the morphology of the solid SiNW that abuts the solid–liquid interface. The probe radius is fixed at 1.2Å. Schematic illustration of one of the corners (arrow). The disordered region is shaded grey. (a, right) Atomic plots of the midsections along the indicated directions showing the distribution of the {113} and {120} family of truncating facets flanking the interface. (b) The in-plane density profile
. (a, middle, inset) The surface plot viewed along the [111] direction showing the morphology of the solid SiNW that abuts the solid–liquid interface. The probe radius is fixed at 1.2Å. Schematic illustration of one of the corners (arrow). The disordered region is shaded grey. (a, right) Atomic plots of the midsections along the indicated directions showing the distribution of the {113} and {120} family of truncating facets flanking the interface. (b) The in-plane density profile  averaged over each liquid bilayer abutting the
averaged over each liquid bilayer abutting the  111
111 interface (shaded strip in a) as a function of the distance z. The arrows indicate peaks consistent with Si segregation within the bilayer. (c) 2D schematic illustrating the interplay between the segregation profile and the interfacial energetics that maintains the local equilibrium at the contact line and the droplet shape, mainly angles α and β, main facet length Λ(111), and truncating facet lengths
interface (shaded strip in a) as a function of the distance z. The arrows indicate peaks consistent with Si segregation within the bilayer. (c) 2D schematic illustrating the interplay between the segregation profile and the interfacial energetics that maintains the local equilibrium at the contact line and the droplet shape, mainly angles α and β, main facet length Λ(111), and truncating facet lengths  and
and  .
.
Surface plot of the solid nanowire sans the the liquid atoms yields insight into the interface morphology (middle panel, Fig. 3a). Clearly, it is not flat. The  111
111 facet is dominant yet it is truncated along the periphery next to the contact line. These truncated regions are 2–4 atomic diameters wide and their length varies non-uniformly along the contact line. Multiple midsections reveal a stepped structure yet they are not smoothly curved; rather they correspond to two distinct orientations inclined at β≈32° and β≈50° to the
facet is dominant yet it is truncated along the periphery next to the contact line. These truncated regions are 2–4 atomic diameters wide and their length varies non-uniformly along the contact line. Multiple midsections reveal a stepped structure yet they are not smoothly curved; rather they correspond to two distinct orientations inclined at β≈32° and β≈50° to the  111
111 sidewall facet which we identify to be the {113} and {120} family of planes. Supplementary Figure S4 reveals a similar morphology for SiNWs with
sidewall facet which we identify to be the {113} and {120} family of planes. Supplementary Figure S4 reveals a similar morphology for SiNWs with  111
111 sidewall facets, with the exception that the truncating facets consist of {113} and {111} family of planes. The six facets alternate between these two inclinations along the contact line, as indicated in the surface plot and the midsections (Fig. 3a, right). The facets are also observed in the doubly capped nanowire system shown in Fig. 1b. The six edge corners where truncating facets intersect are inherently rough as they have to absorb the differences in the facet inclinations, as illustrated schematically. The roughening can also be seen in the surface plot in that the step morphology kinks at the corner edges. The droplet shape is therefore asymmetric as the angles α and β that follow from the Young’s balance along the contact line are shaped by the facet energetics and geometry. The asymmetry is also evident in the two midsections: α≈11° at the {113} truncating facet and changes to α≈15° at the {120} truncating facet.
sidewall facets, with the exception that the truncating facets consist of {113} and {111} family of planes. The six facets alternate between these two inclinations along the contact line, as indicated in the surface plot and the midsections (Fig. 3a, right). The facets are also observed in the doubly capped nanowire system shown in Fig. 1b. The six edge corners where truncating facets intersect are inherently rough as they have to absorb the differences in the facet inclinations, as illustrated schematically. The roughening can also be seen in the surface plot in that the step morphology kinks at the corner edges. The droplet shape is therefore asymmetric as the angles α and β that follow from the Young’s balance along the contact line are shaped by the facet energetics and geometry. The asymmetry is also evident in the two midsections: α≈11° at the {113} truncating facet and changes to α≈15° at the {120} truncating facet.
The presence of nanometer-wide stable truncating facets is consistent with recent reports in several semiconducting nanowire systems, including the Au–Si system43,44,45. Limitations of the model system notwithstanding, such a complex 3D morphology composed of multiple families of truncating facets is similar to that recently reported in [0001] sapphire nanowires43, suggesting that this is perhaps a general feature during VLS growth. For the specific case of Si, it is interesting that the {113} and {111} facets routinely decorate the sidewalls during the complex sawtooth faceting in much larger diameter  111
111 SiNWs14,36, suggesting that they perhaps nucleate first at the solid–liquid interface.
SiNWs14,36, suggesting that they perhaps nucleate first at the solid–liquid interface.
Nanowire growth kinetics
The heterogeneous nature of interface facets has important ramifications for nanowire growth, which we now study computationally. We simplify the catalysis by directly abstracting individual silicon atoms onto the droplet surface to a prescribed level of supersaturation, and employ classical MD simulations to study the non-equilibrium response of the entire droplet–SiNW–substrate system (Supplementary Methods). Figure 4 shows the structural evolution at and around the interface as well as the in-plane density profile  for a droplet with initial composition XSi=0.48 (ΔT≈36 K). The initial growth stage for t<0.4 ns is marked by layer-by-layer crystallization on the truncating facets. The circumferential growth is likely aided by their small widths and the proximity to the Si-rich contact line (circled, Fig. 4a). We see 1D lengthening of kinks associated with the stepped structure of these facets. Interestingly, the growth occurs preferentially away from the corner edges implying that they favour dissolution (for details, see Supplementary Movie 1). The extent of truncation changes due to their growth, consistent with the oscillatory growth wherein the facet widths decrease between bilayer additions on the main facet43,44,45. We also see smaller clusters of 2–3 Si atoms that continually form and dissolve on the main facet yet the density profile normal to the interface remains unchanged (bottom row), that is, the crystallization is limited to the truncating facets.
for a droplet with initial composition XSi=0.48 (ΔT≈36 K). The initial growth stage for t<0.4 ns is marked by layer-by-layer crystallization on the truncating facets. The circumferential growth is likely aided by their small widths and the proximity to the Si-rich contact line (circled, Fig. 4a). We see 1D lengthening of kinks associated with the stepped structure of these facets. Interestingly, the growth occurs preferentially away from the corner edges implying that they favour dissolution (for details, see Supplementary Movie 1). The extent of truncation changes due to their growth, consistent with the oscillatory growth wherein the facet widths decrease between bilayer additions on the main facet43,44,45. We also see smaller clusters of 2–3 Si atoms that continually form and dissolve on the main facet yet the density profile normal to the interface remains unchanged (bottom row), that is, the crystallization is limited to the truncating facets.
(a–e) Atomic configurations in the vicinity of the interface subject to a supersaturated droplet with initial composition XSi=0.48. (top row) Liquid bilayer abutting the droplet–SiNW interface, and (middle row) top and side views of the solid nanowire. The light- and dark-blue colours indicate crystallized Si atoms on the truncating and main facets, respectively. The instantaneous supersaturation is indicated on the top of each panel. The circled region in a highlights an instance of preferential growth at the centre of the truncating facet while the circled region in b indicates precursor fluctuations that lead to the formation of a critical nucleus on the main facet. Details can be seen in Supplementary Movie 1. The bottom of each panel shows in-plane density profile  . (f) Schematic illustration of the evolution of the interface before and during growth via step flow. See text for discussion. (g) Temporal evolution of crystallized net Si volume (red circles) composed of crystallization on the edge and main facet (light and dark blue circles, respectively), as well as the droplet composition (solid green squares). The linear fit (solid red line) used to extract the layer growth rate due to post-nucleation step flow on the main facet. (h) Size of the nuclei observed on the main facet. The red curve is the evolution of the critical nucleus as indicated, while the other curves correspond to evolution of the smaller peripheral nuclei.
. (f) Schematic illustration of the evolution of the interface before and during growth via step flow. See text for discussion. (g) Temporal evolution of crystallized net Si volume (red circles) composed of crystallization on the edge and main facet (light and dark blue circles, respectively), as well as the droplet composition (solid green squares). The linear fit (solid red line) used to extract the layer growth rate due to post-nucleation step flow on the main facet. (h) Size of the nuclei observed on the main facet. The red curve is the evolution of the critical nucleus as indicated, while the other curves correspond to evolution of the smaller peripheral nuclei.
The t=0.8 ns configuration plotted in Fig. 4b shows that, barring the corner edges, the growth has advanced by almost a full layer on the truncating facets. The droplet is still supersaturated and it now drives the nucleation on the main facet. It is worth pointing out that structurally the growth on the truncating facets and on the main facet is essentially independent, as a 2D island reaching the edge of one facet has no nearest neighbours with an island on the adjacent facet. They require the influence of the much weaker next nearest-neighbor interactions to extend across. We observe early stages of classical 2D layer-by-layer growth wherein several nuclei form and attempt to overcome the nucleation barrier. Observe that the nuclei form slightly away from the centre of the main facet and their location is correlated with the corner edges. The disorder together with the enhanced Si segregation at the contact line likely aids the nucleation on the main facet. The diffusion along the droplet surface is indeed slower yet the bulk liquid can easily provide a pathway. The precursor cluster that eventually becomes supercritical is circled in Fig. 4b. Figure 4c–e reveals the key features of the subsequent bilayer growth on the main facet, that is, it remains layer-by-layer and dominated by a single growing nucleus.
The density profiles evolve accordingly. The intensity of the two silicon peaks increases at the expense of the Au peak. A sharp peak in the adjacent liquid layer indicates build-up of Si while the third layer becomes Au rich (arrow in Fig. 4d). Evidently, Au diffuses rapidly away from the interface. At t=2 ns, the first layer has not completely formed yet the step flow has slowed down due to the low supersaturation left in the droplet, XSi=0.463. Note that there is negligible growth at the edge corners (circled, Fig. 4e). We see Au and liquid Si within the mostly crystallized Si layer, although the liquid Si atoms exhibit in-plane order. Eventually, some of the Au atoms diffuse away into the liquid droplet and aid further crystallization (not shown).
We quantify these observations by monitoring the crystallized volume at the interface V(t) and the droplet composition XSi(t) (Fig. 4g). Crystallization kinetics on the truncating and main facets are plotted separately. Initially for t<1 ns the increase in V(t) is entirely due to crystallization on the truncating facets. A linear fit yields a growth rate of v≈10.5 cm s−1. The growth is instantaneous indicative of a small nucleation barrier. Although the barrier can be artificially reduced due to the larger supersaturations in the computations, note that the Si-rich contact line is an easy source for Si adatoms. Also, as in the experiments, the facet widths rarely exceed a few nanometers such that the critical nuclei sizes are likely larger than the facet widths46. The circumferential 1D growth is also aided by the stepped structure of these facets and we quantify this effect by extracting Si adatom enthalpies Had on the {111} and the {113} family planes (Methods). Not surprisingly, the close-packed {111} facets have a significantly higher enthalpy than the stepped {113} facets, ΔHad≈2.0 eV per adatom. The adatom diffusivity on the {113} facets is the suppressed as the atom makes fewer diffusion hops before another atom crystallizes in close proximity. The adatoms can readily form clusters and therefore lower the nucleation barrier, which in turn can induce rapid 1D growth. The role of such structural effects in introducing kinetic anisotropies during faceted crystal growth is well established47.
At t≈1.0 ns, the growth rate on the truncating facets decreases, either due to site saturation or the onset of the oscillatory growth mode. The growth on the main facet, non-existent so far, dramatically increases. This is indicative of the growth of a supercritical nuclei and is again consistent with our qualitative observations (Figs 4c–e). To ascertain whether the growth is layer-by-layer or kinetically rough, we monitor the size evolution of the visibly identifiable nuclei on the main facet (Fig. 4h). We see a dramatic increase in the size of one of the nuclei while the other nuclei fluctuate about a relatively smaller size (<35 atoms). At longer times t≈10 ns, one of these smaller nuclei shrinks while the other two persist without any appreciable increase in size. Clearly, one 2D island dominates the layer growth and the encompassing steps are the main contributions to surface roughening. Moreover, the step orientations reflect the symmetry of the growth direction while that is not the case for the fluctuating subcritical islands. The size evolution shows that even though the driving force is larger than that in experiments, kinetic roughening or the increase in step density resulting from growth on the main facet is non-existent48. The persistence of the subcritical nuclei can signify that the supersaturation is on the borderline between single nucleus and polynuclear growth; then at lower supersaturations, we expect the growth of the nanowires to be universally by the single nucleus mechanism. Following nucleation, the growth is steady state as the volume increases and composition decreases. A linear fit in this regime yields the overall growth velocity, v≈6.3 cm s−1. The growth rate also includes contributions from the truncating facets. Subtracting the former from the overall growth rate yields the step velocity on the main facet, vs≈3 cm s−1. Figure 4f is a schematic summary of the morphological features implicated in the overall growth.
Effect of supersaturation
To make contact with experimental scales, we quantify the nucleation barrier on the main facet by performing computations with decreasing initial supersaturation. Representative results for XSi=0.463 (ΔT≈5.4 K) are shown in Fig. 5. The truncating facets again grow rapidly and circumferentially (Fig. 5a). The growth is instantaneous yet slower, and the growth on the main facet is non-existent (Fig. 5b). The crystallization is again preferentially at the centres of the truncating facets, presumably due to a 1D interplay between crystallization at the facet centre and melting at the rough corner edges (Supplementary Movie 2). At t≈3.0 ns, we see transient crystallization of subcritical nuclei on the main facet yet there is no supersaturation left for their growth. Then, XSi=0.463 represents a critical initial composition below which there is no nucleation on the main facet, that is, nanowire growth can only occur at larger supersaturations.
Top row: atomic configurations of the solid nanowire growth front subject to an initial droplet supersaturation of XSi=0.463. The elapsed time and the current supersaturation are also indicated. For details, see Supplementary Movie 2. Bottom: the temporal evolution of the crystallized volume V(t) and the composition within the droplet, XSi(t). Inset: evolution of the crystallized volume on the main and truncating facets.
Increasing the initial composition in the range XSi≥0.485 (ΔT≥45 K) decreases the dead zone that precedes the nucleation on the main facet. Multiple stable islands contribute to the growth of a single layer such that the step density increases and there is a gradual transition to a kinetically roughened regime, wherein the second bilayer begins to crystallize before the complete growth of the first bilayer. This is evident in configurations for XSi=0.485 shown in Supplementary Fig. S5. Several Au atoms remain confined within the first layer. They likely retard the step flow and that triggers the onset of a 3D growth mode. Midsections reveal additional Si diffusion down the sidewalls that further aids the absorption of the supersaturation (inset).
Figure 6a shows the driving force dependence of the overall growth velocities for varying supersaturations. The velocities are linear fits to the initial crystallization rates. In the small driving force limit XSi≤0.48 (ΔT<36 K), the velocity increases rapidly. Results of similar computations on a planar Si(111)–AuSi interface are also plotted for comparison (see Methods). Crystallization at the planar interface entails homogeneous nucleation and is always slower at comparable driving forces, suggesting a higher nucleation barrier. Separate plots for the contributions of the truncating and main facets are plotted in Fig. 6b. The velocity of the truncating facets increases linearly with supersaturation and indicates that its growth is not nucleation controlled, at least for undercoolings as low as ΔT=5.4 K. The average kinetic coefficient is ≈3.0 mm/(s K) and is of the order of kinetic coefficients in binary alloys49.
(a) The extracted linear growth velocity v=[1/(πR2)dV/dt] of the nanowire as a function of the initial supersaturation,  . The equivalent undercooling based on the phase diagram in Fig. 1a is also indicated. For comparison, growth velocity of a planar Si(111)–AuSi solid–liquid interface is plotted (dotted line) as a function of initial supersaturation ΔX(R=∞) within the AuSi phase. The grey shaded areas represent kinetically roughened growth regime induced by large supersaturations. (b) Expanded view of the small supersaturation growth data corresponding to the boxed region in a. The contributions of the two classes of facets and the relevant curve fits are shown as separate plots.
. The equivalent undercooling based on the phase diagram in Fig. 1a is also indicated. For comparison, growth velocity of a planar Si(111)–AuSi solid–liquid interface is plotted (dotted line) as a function of initial supersaturation ΔX(R=∞) within the AuSi phase. The grey shaded areas represent kinetically roughened growth regime induced by large supersaturations. (b) Expanded view of the small supersaturation growth data corresponding to the boxed region in a. The contributions of the two classes of facets and the relevant curve fits are shown as separate plots.
The crystallization rate on the main facet increases non-linearly with the supersaturation signifying classical nucleation-controlled growth. It is possible that at much smaller driving forces the growth is linear as the finite facet size requires equilibrium between melting and nucleation33, although it is not clear if this applies to a fully faceted interface. Accordingly, we fit the variation to a functional form  , where the exponential factor is the probability that a spontaneous fluctuation will result in a critical nucleus, and v0 is the 2D growth rate of the supercritical nucleus that is almost independent of the supersaturation. The nucleation barrier varies as ΔG
, where the exponential factor is the probability that a spontaneous fluctuation will result in a critical nucleus, and v0 is the 2D growth rate of the supercritical nucleus that is almost independent of the supersaturation. The nucleation barrier varies as ΔG 1/ΔX and the exponential fit to the extracted velocities yields a value ΔG=4 meV/ΔX at T=873 K. A similar fit to the planar interface data yields a barrier that is 63% larger and is consistent with estimates based on nucleation of a 2D island (Supplementary Note 1). At larger driving forces characterized by kinetically rough 3D growth, the velocity increases almost linearly with supersaturation. The plot also shows results of computations on undersaturated droplets (
1/ΔX and the exponential fit to the extracted velocities yields a value ΔG=4 meV/ΔX at T=873 K. A similar fit to the planar interface data yields a barrier that is 63% larger and is consistent with estimates based on nucleation of a 2D island (Supplementary Note 1). At larger driving forces characterized by kinetically rough 3D growth, the velocity increases almost linearly with supersaturation. The plot also shows results of computations on undersaturated droplets ( ). At small driving forces, the nucleation and growth dynamics associated with the melting kinetics is completely reversed; the truncating facets melt first as the energy of formation of a vacancy on these facets is lower, and eventually the main facet melts preferentially from the corner edges. The layer-by-layer melting velocities are smaller compared with those during growth due to the different morphological features implicated in the two processes, which by itself is important as the growth is a steady state between crystallization and melting kinetics33,46.
). At small driving forces, the nucleation and growth dynamics associated with the melting kinetics is completely reversed; the truncating facets melt first as the energy of formation of a vacancy on these facets is lower, and eventually the main facet melts preferentially from the corner edges. The layer-by-layer melting velocities are smaller compared with those during growth due to the different morphological features implicated in the two processes, which by itself is important as the growth is a steady state between crystallization and melting kinetics33,46.
Mechanistic insight during growth
Maps of atomic displacements over prescribed time intervals corroborate the observed trends. Figure 7a–d shows a t=0.5–1.0 ns map for select Si and Au atoms within the XSi=0.48 computation. The displacements correspond to newly crystallized Si atoms (Si map) and Au atoms initially at the interface (Au map). The 2D step flow results in linear decrease of the droplet composition from XSi=0.474 to XSi=0.469 (Fig. 4g). The Si crystallization is mostly from adjacent bilayers and driven by displacement chains. Surprisingly, we also see long-range fast diffusion from as far as the droplet surface (Fig. 7a). The flux is mostly through the amorphous bulk of the droplet and we see negligible surface diffusion (Fig. 7b). The step flow is aided by Au diffusion into the droplet (Fig. 7c) and also along the interface directed away from the moving steps (Fig. 7d). We see evidence for both short-range atomic hops into adjacent liquid bilayer and long-range diffusion towards the droplet surface. The diffusion is markedly along the bulk and also towards the nanowire sidewalls; the latter represents a direct link between nanowire growth and Au decoration on the sidewalls.
Cumulative displacement maps for select (a,b) Si and (c,d) Au atoms over a 0.5-ns time interval following nucleation, for initial composition XSi=0.48. Both side (a,c) and top (b,d) are shown. The Si displacements correspond to crystallized atoms within the time interval. The Au displacements are for atoms initially at the solid–liquid interface. Within each map, the arrow lengths are proportional to the net displacement. Atomic configurations of midsections (side views) and bilayers (top views) are superposed within the maps to facilitate visual location of the crystallizing Si atoms (shaded blue).
Discussion
The atomic-scale structure and dynamics during VLS growth of SiNWs is clearly sensitive to segregation at the surface and the droplet–nanowire interface. The robust surface layer is expected to be stable under reactor conditions where the droplet is exposed to the precursor gas atmosphere. The Au submonolayer together with the inhibited surface diffusion renders the droplet surface in immediate contact with the vapour sufficiently Au rich to drive the precursor breakdown over large pressure and temperature ranges. The heterogeneously faceted interface morphology impacts all aspects of the growth process44 and requires reconsideration of current growth models. The truncating facets induce variations in the Si segregation and droplet morphology along the contact line. The relative disordered corner edges impact interface nucleation and growth. They likely serve as efficient sources of steps for rapid crystallization on the truncating facets, analogous to the screw dislocation-based spiral crystal growth50. At much lower supersaturations, the dynamics may involve a competition between nucleation at the facet centres and melting at the edge corners. Nevertheless, the barrier is clearly much smaller than that on the main facet. The latter is clearly the rate-limiting event. The growth of the truncating facets also decreases the extent of these ordered regions, and they can facilitate mass transfer from the Si-rich contact line onto the main facet and thereby lower the barrier compared with an equivalently supersaturated planar Si–AuSi interface; the computations show that this is indeed the case. The extracted nucleation barrier ΔG allows us to establish nucleation- and catalysis-controlled regimes. Under typical growth conditions, the atomic incorporation rate is of the order of a single Si atom per millisecond (Supplementary Note 2). Equating this incorporation rate to the nucleation-controlled growth rate, the extracted barrier ΔG=4 meV/ΔX yields a supersaturation of  for isothermal nanowire growth at T=873 K, or a critical undercooling of
for isothermal nanowire growth at T=873 K, or a critical undercooling of  K. The growth is then nucleation controlled for
K. The growth is then nucleation controlled for  and catalysis controlled otherwise. Solidification from melts typically occurs at undercooling of less than a Kelvin51, and if that is the case during VLS growth of nanowires, it must be nucleation controlled. We should emphasize that as the barrier is expected to be nanowire size dependent, the interplay between nucleation and catalysis can be quantitatively different at larger sizes and is a focus of future studies.
and catalysis controlled otherwise. Solidification from melts typically occurs at undercooling of less than a Kelvin51, and if that is the case during VLS growth of nanowires, it must be nucleation controlled. We should emphasize that as the barrier is expected to be nanowire size dependent, the interplay between nucleation and catalysis can be quantitatively different at larger sizes and is a focus of future studies.
An interesting coda is the transition to kinetically rough growth mode at larger supersaturations, where the step flow is increasingly limited by Au diffusion away from the step edges. The inter-island Au atoms are stable over the MD time scales (<10 ns), and although we cannot rule out their slow diffusion out of the nanowire eventually, there is growing evidence of catalyst particle trapping within the as-grown nanowires52. Detailed understanding of this regime opens up the possibility of doping nanowires to non-equilibrium compositions during their synthesis17, a scenario worthy of detailed explorations by itself.
Methods
Model system
We use classical inter-atomic potentials to model the eutectic AuSi system. Hydrogenation effects are ignored due to hydrogen desorption and Au surface passivation at these elevated temperatures. The potentials can be a limitation as they represent approximations to the electronic degrees of freedom. Although the AEAM model system is fit to several experimental and first-principles data and is based on established frameworks for both pure Au and Si, its ability to make quantitative predictions, notably interface properties, may be limited. For example, the pure Au potential underestimates the liquid free energies41, whereas the pure Si potential is limited in describing surface reconstructions on crystal facets53. Nevertheless, validation studies presented here show that the predictions of the model potential are in agreement with several alloy properties. The AEAM framework reproduces the primary feature of the binary phase diagram, that is, a low melting eutectic. The noteworthy deviations are an underestimation of the eutectic temperature TE (590 K compared with 636 K) and overestimation of the eutectic composition  (31% compared with 19%). We have also performed contact angle studies of eutectic and hypereutectic AuSi droplets on Si(111) surface using semi-grandcanonical Monte–Carlo (SGMC) simulations (Supplementary Methods). The final configuration of a 2R=10 nm size droplet on a Si(111) surface at the growth temperature is shown in Supplementary Fig. S6. The contact angle θ=143° is close to the experimental value54 of θ=136°.
(31% compared with 19%). We have also performed contact angle studies of eutectic and hypereutectic AuSi droplets on Si(111) surface using semi-grandcanonical Monte–Carlo (SGMC) simulations (Supplementary Methods). The final configuration of a 2R=10 nm size droplet on a Si(111) surface at the growth temperature is shown in Supplementary Fig. S6. The contact angle θ=143° is close to the experimental value54 of θ=136°.
Equilibrium liquidus concentration for 2R=10 nm SiNW
The initial structure consists of a pristine 2R=10 nm  111
111 SiNW with {110} or {112} family of lateral facets capped by two AuSi particles. The Si(111)–AuSi interfaces are initially flat. The SiNW length is ≈8 nm, large enough such that the interfaces do not interact. The atomic structure of the particles is generated randomly at a density corresponding to liquid AuSi and the initial composition is hypoeutectic, XSi=0.25. The entire system consists of ~64,000 atoms and is relaxed using SGMC computations. A local order parameter is employed to demarcate the liquid and solid Si atoms55. The order parameter is an extension of the pure Si case based on the local 3D structure around each Si atom. Additional validation of the surface segregation is done using charge gradient corrections to the Au–Au interactions that correctly reproduces the liquid Au surface tension (Supplementary Methods).
SiNW with {110} or {112} family of lateral facets capped by two AuSi particles. The Si(111)–AuSi interfaces are initially flat. The SiNW length is ≈8 nm, large enough such that the interfaces do not interact. The atomic structure of the particles is generated randomly at a density corresponding to liquid AuSi and the initial composition is hypoeutectic, XSi=0.25. The entire system consists of ~64,000 atoms and is relaxed using SGMC computations. A local order parameter is employed to demarcate the liquid and solid Si atoms55. The order parameter is an extension of the pure Si case based on the local 3D structure around each Si atom. Additional validation of the surface segregation is done using charge gradient corrections to the Au–Au interactions that correctly reproduces the liquid Au surface tension (Supplementary Methods).
Equilibrium studies on isolated AuSi particles and thin films
Density profiles of the equilibrated droplets are averaged over bilayer thick spherical shells around the centre of mass of the droplet, and the ensemble average is over 1,000 different atomic configurations. To quantify the effect of droplet surface curvature, the density profiles are also extracted for thin films. We employ an initially liquid film of thickness H=10 nm, free at both surfaces and periodic in the film plane. SGMC simulations are used to equilibrate the system. Supplementary Figure S1 shows the equilibrated configuration for a 27,000-atom simulation and the corresponding normalized density profiles as a function of a distance from the surface h − H extracted at the bulk eutectic composition and temperature, XSi=0.31 and TE=590 K. Layering of both Au and Si is clearly visible and a Au monolayer decorates the Si-rich surface. Supplementary Figure S1 also shows the density profiles for thin films at the growth temperature selected for this study (T=873 K) and for size-corrected liquidus point, (T=873 K, XSi=0.46). Comparison with the profile at the eutectic point clearly shows a decrease in the thickness of the solid surface layer with increasing temperature and Si supersaturation.
Au and Si diffusivities within the droplet
MD simulations are performed on the equilibrated droplets to extract the ensemble-averaged MSD of both Au and Si (Supplementary Methods). A net simulation time of a few tens of picoseconds is sufficient to extract statistically meaningful data. For each atomic species at an initial distance r from the mass centre of the particle, the MSD increases linearly with time and the slope yields the diffusivity D(r). For atoms in the bulk and on the surface, the slope is different. The MSDs for Au and Si atoms initially at the surface of the 2R=10 nm Au54Si46 droplet are plotted in Supplementary Fig. S3. The computations on bulk eutectic AuSi alloy yield a diffusivity of DAu=1.54 × 10–9 m2 s−1 at 873 K; the value is in excellent agreement with past experiments on Au diffusivity in eutectic AuSi alloys56, DAu≈1.7 × 10−9 m2 s−1.
Equilibrium state of the droplet–nanowire–substrate system
The initial configuration is generated by taking one half of the double droplet configuration and placing it on a (16.0 nm × 15.4 nm) Si(111) substrate. The dimensions of the substrate are the same as in the contact angle studies. The bottom bilayer is fixed. The exposed area is decorated by a Au monolayer with a structure similar to the classical  monolayer structure56, and the entire system is equilibrated using canonical Monte–Carlo simulations followed by MD simulations.
monolayer structure56, and the entire system is equilibrated using canonical Monte–Carlo simulations followed by MD simulations.
Nanowire growth kinetics
The catalytic incorporation of individual Si atoms takes place over much larger time scales, and we expect the interface growth to be orders of magnitude faster. Therefore, there is no additional Si flux imposed on the droplet during the course of the MD computations, and we investigate the effect of an initially prescribed driving force that evolves in a manner consistent with nucleation-limited growth. Although the supersaturation is arbitrary and likely larger compared with the critical supersaturation in experiments, a systematic variation of the driving force allows us to extract the key features associated with the near-equilibrium behaviour of the nanowire during its growth. The supersaturation for growth is generated by attempting to place Si atoms on the surface within an MD simulation and then accepting the move if an Au atom is within a nearest-neighbor distance. Alternate procedure wherein the droplet is supersaturated by directly introducing atoms within an isolated droplet have negligible effect on the ensuing dynamics; the segregation at the surface and interface is fairly robust. The MD simulations employed are same as those for diffusivity calculations.
Si(111)–AuSi interface kinetics and step energetics
The simulation cell consists of a crystalline Si(111) substrate (8,640 atoms) abutting a AuSi alloy (34,560 atoms). The dimensions of simulation cell is 14.14a0 × 14.69a0 × 25.97a0, where a0 is the lattice parameter at 873 K. The initial AuSi alloy with the desired concentration is randomly structured and MD simulations are performed at 873 K. Periodic boundary conditions are applied along the in-plane directions with free surfaces normal to the interface. The Si(111) substrate is initially fixed and the AuSi alloy is relaxed for 0.1 ns. The planar nucleation/melting simulations are carried by fixing the bottom bilayer of the substrate. The crystalline and liquid Si atoms are identified using the local order parameter described earlier. The simulation cell for extracting the step enthalpies is similar; it is 19.80a0 × 19.60a0 × 10.39a0 in size and consists of a Si(111) slab (16,128 atoms) and a AuSi alloy slab (16,128 atoms). The composition of the initial AuSi alloy is XSi=0.46 and it is relaxed using isobaric, isothermal MD simulations (Supplementary Methods). The simulation cell is fully periodic so that the cell consists of two Si(111)–AuSi interfaces. The dimension normal to normal to the free surface adjusts to maintain zero pressure while two in-plane dimensions normal to  and
and  are fixed. Following relaxation (0.3 ns), the total potential energy U1 is averaged over 0.1 ns. Single bilayer steps are created on both surfaces of Si slab while preserving the atoms in the Si(111) crystal and liquid AuSi. The steps are oriented along the index (112) direction. The total potential potential energy is recorded as U2 and the difference σ=(U2−U1)/L=0.16 eV/nm, where L is the total step length.
are fixed. Following relaxation (0.3 ns), the total potential energy U1 is averaged over 0.1 ns. Single bilayer steps are created on both surfaces of Si slab while preserving the atoms in the Si(111) crystal and liquid AuSi. The steps are oriented along the index (112) direction. The total potential potential energy is recorded as U2 and the difference σ=(U2−U1)/L=0.16 eV/nm, where L is the total step length.
Si adatom energetics on truncating and main facets
Canonical MD simulations are performed at temperature T=1 K. Each computational cell consists of an unreconstructed crystal Si slab, free at both both surfaces and periodic in the slab plane. The dimensions of simulation cells are (7.07a0 × 9.38a0 × 6.63a0) and (7.07a0 × 7.35a0 × 5.20a0) for (113) and (111) surfaces and contains 3,520 and 2,160 atoms, respectively. The slabs are relaxed for 10 ps. A single Si atom is placed within the interaction range on each of the surfaces and relaxed for another 10 ps. All possible positions with maximum nearest neighbours were simulated to obtain the lowest potential energy. The maximum potential energy difference before and after addition of the Si adatom is recorded as the Si adatom enthalpy Had. The extracted enthalpies are −5.4 eV per atom and −3.5 eV per atom for the (113) and (111) surfaces, respectively. Note that the stepped structure of unreconstructed (113) surfaces can be interpreted as (111) steps separated by single atomic rows of (001) terraces. The {120} facets are also stepped and we therefore expect similar trends.
Additional information
How to cite this article: Wang, H. et al. Atomistics of vapour–liquid–solid nanowire growth. Nat. Commun. 4:1956 doi: 10.1038/ncomms2956 (2013).
References
Wagner, R. S. & Ellis, W. C. Vapor-liquid-solid mechanism of single crystal growth. Appl. Phys. Lett. 4, 89–90 (1964).
Givargizov, E. I. & Chernov, A. A. Growth rate of crystal whiskers grown by the vapor-liquid-crystal mechanism and role of surface energy. Kristallografiya 18, 147–153 (1973).
Wu, Y. Xiang, J. Yang, C. Lu, W. & Lieber, C. M. Single-crystal metallic nanowires and metal/semiconductor nanowire heterostructures. Nature 430, 61–65 (2004).
Schmidt, V. Senz, S. & Gösele, U. Diameter-dependent growth direction of epitaxial silicon nanowires. Nano Lett. 5, 931–935 (2005).
Schmidt, V. Wittemann, J. V. Senz, S. & Gösele, U. Silicon nanowires: A review on aspects of their growth and their electrical properties. Adv. Mater. 21, 2681–2702 (2009).
Ross, F. M. Controlling nanowire structures through real time growth studies. Rep. Prog. Phys. 73, 114501 (2010).
Lieber, C. M. One-dimensional nanostructures: Chemistry, physics and applications. Solid State Commun. 107, 607–616 (1998).
Lieber, C. M. The incredible shrinking circuit. Sci. Am. 285, 58–64 (2001).
Lieber, C. M. Nanoscale science and technology: Building a big future from small things. MRS Bull. 28, 486–491 (2003).
Baker, S. P. Vinci, R. P. & Arias, T. Elastic and anelastic behavior of materials in small dimensions. MRS Bull. 27, 26–29 (2002).
Samuelson, L. et al. Semiconductor nanowires for novel one-dimensional devices. Physica E 21, 560–567 (2004).
Roper, S. M. et al. Steady growth of nanowires via the vapor-liquid-solid method. J. Appl. Phys. 102, 034304 (2007).
Ross, F. M. Tersoff, J. & Reuter, M. C. Sawtooth faceting in silicon nanowires. Phys. Rev. Lett. 95, 146104 (2005).
Schmidt, V. Senz, S. & Gösele, U. Diameter dependence of the growth velocity of silicon nanowires synthesized via the vapor-liquid-solid mechanism. Phys. Rev. B 75, 045335 (2007).
Nayfeh, O. M. Antoniadis, D. A. Boles, S. Ho, C. & Thompson, C. V. Formation of single tiers of bridging silicon nanowires for transistor applications using vapor-liquid-solid growth from short silicon-on-insulator sidewalls. Small 5, 2440–2444 (2009).
Schwalbach, E. J. & Voorhees, P. W. Doping nanowires grown by the vapor-liquid-solid mechanism. Appl. Phys. Lett. 95, 063105 (2009).
Madras, P. Dailey, E. & Drucker, J. Kinetically induced kinking of vapor-liquid-solid grown epitaxial Si nanowires. Nano Lett. 9, 3826–3830 (2009).
Irrera, A. Pecora, E. F. & Priolo, F. Control of growth mechanisms and orientation in epitaxial Si nanowires grown by electron beam evaporation. Nanotechnology 20, 135601 (2009).
Givargizov, E. I. Fundamental aspects of VLS growth. J. Cryst. Growth 31, 20–30 (1975).
Dubrovskii, V. G. & Sibirev, N. V. Growth rate of a crystal facet of arbitrary size and growth kinetics of vertical nanowires. Phys. Rev. E 70, 031604 (2004).
Schwarz, K. W. & Tersoff, J. From droplets to nanowires: Dynamics of vapor-liquid-solid growth. Phys. Rev. Lett. 102, 206101 (2009).
Dubrovskii, V. G. et al. Role of nonlinear effects in nanowire growth and crystal phase. Phys. Rev. B 80, 205305 (2009).
Schwarz, K. W. Tersoff, J. Kodambaka, S. Chou, Y. C. & Ross, F. M. Geometrical frustration in nanowire growth. Phys. Rev. Lett. 107, 265502 (2011).
Schwarz, K. W. & Tersoff, J. Elementary processes in nanowire growth. Nano Lett. 11, 316–320 (2011).
Kodambaka, S. Tersoff, J. Reuter, M. C. & Ross, F. M. Diameter-independent kinetics in the vapor-liquid-solid growth of Si nanowires. Phys. Rev. Lett. 96, 096105 (2006).
Kim, B. J. et al. Kinetics of individual nucleation events observed in nanoscale vapor-liquid-solid growth. Science 322, 1070–1073 (2008).
Kim, B. J. et al. Determination of size effects during the phase transition of a nanoscale Au-Si eutectic. Phys. Rev. Lett. 103, 155701 (2009).
Perea, D. E. et al. Three-dimensional nanoscale composition mapping of semiconductor nanowires. Nano Lett. 6, 181–185 (2006).
Perea, D. E. et al. Direct measurement of dopant distribution in an individual vapour-liquid-solid nanowire. Nat. Nanotech. 4, 315–319 (2009).
Hemesath, E. R. et al. Catalyst incorporation at defects during nanowire growth. Nano Lett. 12, 167–171 (2012).
Lee, S.-H. Stephens, J. A. & Hwang, G. S. On the nature and origin of Si surface segregation in amorphous AuSi alloys. J. Phys. Chem. C 114, 3037–3041 (2010).
Haxhimali, T. Buta, D. Asta, M. Voorhees, P. W. & Hoyt, J. J. Size-dependent nucleation kinetics at nonplanar nanowire growth interfaces. Phys. Rev. E 80, 050601(R) (2009).
Ryu, S. & Cai, W. Molecular dynamics simulations of gold-catalyzed growth of silicon bulk crystals and nanowires. J. Mater. Res. 26, 2199–2206 (2011).
Dongare, A. M. Neurock, M. & Zhigilei, L. V. A new angular-dependent embedded-atom-method potential for atomistic simulations of metal-covalent systems. Phys. Rev. B 80, 184106 (2009).
David, T. Buttard, D. Schülli, T. Dallhuin, F. & Gentile, P. Structural investigation of silicon nanowires using GIXD and GISAXS: evidence of complex saw-tooth faceting. Surf. Sci. 602, 2675–2680 (2008).
Hannon, J. B. Kodambaka, S. Ross, F. M. & Tromp, R. M. The influence of the surface migration of gold on the growth of silicon nanowires. Nature 440, 69–71 (2006).
Herring, C. Some theorems on the free energies of crystal surfaces. Phys. Rev. 82, 87–93 (1951).
Liang, H. Y. Upmanyu, M. & Huang, H. C. Size dependent elasticity of nanowires: Non-linear effects. Phys. Rev. B 71, 241403(R) (2005).
Shpyrko, O. G. et al. Surface crystallization in a liquid AuSi alloy. Science 313, 77–80 (2006).
Webb, E. B. III & Grest, G. S. Liquid/vapor surface tension of metals: Embedded atom method with charge gradient corrections. Phys. Rev. Lett. 86, 2066–2069 (2001).
Lelay, G. L. The Au/Si(111) interface: Growth mode, energetics, structural and electronic properties. J. Cryst. Growth 54, 551–557 (1981).
Oh, S. H. et al. Oscillatory mass transport in vapor-liquid-solid growth of sapphire nanowires. Science 330, 489–493 (2010).
Wen, C.-Y. et al. Periodically changing morphology of the growth interface in Si, Ge, and GaP nanowires. Phys. Rev. Lett. 107, 025503 (2011).
Gamalski, A. D. Ducati, C. & Hofmann, S. Cyclic supersaturation and triple phase boundary dynamics in germanium nanowire growth. J. Phys. Chem. C 115, 4413–4417 (2011).
Rohrer, G. S. Rohrer, C. L. & Mullins, W. W. Nucleation energy barriers for volume conserving shape changes of crystals with nonequilibrium morphologies. J. Am. Ceram. Soc. 84, 2099–2104 (2001).
Zepeda-Ruiz, L. A. Maiti, A. Gee, R. Gilmer, G. H. & Weeks, B. L. Size and habit evolution of PETN crystals—a lattice Monte Carlo study. J. Cryst. Growth 291, 461–467 (2006).
Jackson, K. A. & Gilmer, G. H. Crystal growth and materials. Faraday Disc. Chem. Soc. 61, 53–61 (1976).
Hoyt, J. J. Asta, M. & Karma, A. Atomistic simulation methods for computing kinetic coefficient in solid-liquid systems. Interface Sci. 10, 181–189 (2002).
Burton, W. K. Cabrera, N. & Frank, F. C. The growth of crystals and the equilibrium structure of their surfaces. Phil. Trans. R Soc. Lond. A 243, 299–358 (1951).
Woodruff, D. P. The Solid-Liquid Interface Cambridge Univ. Press (1973).
Moutanabbir, O. et al. Colossal injection of catalyst atoms into silicon nanowires. Nature 496, 78–82 (2013).
Li, X.-P. Chen, G. Allen, P. B. & Broughton, J. Q. Energy and vibrational spectrum of the Si(111) (7 × 7) surface from empirical potentials. Phys. Rev. B 38, 3331–3341 (1988).
Ressel, B. Prince, K. C. Heun, S. & Homma, Y. Wetting of Si surfaces by Au-Si liquid alloys. J. Appl. Phys. 93, 3886–3892 (2003).
Buta, D. Asta, M. & Hoyt, J. J. Atomistic simulation study of the structure and dynamics of a faceted crystal-melt interface. Phys. Rev. E 78, 031605 (2008).
Bruson, A. & Gerl, M. Diffusion coefficient of 195Au in the liquid Au0.81Si0.19 alloy. J. Appl. Phys. 53, 3616–3619 (1982).
Nagao, T. et al. Structural phase transitions of : phase transitions in domain-wall configurations. Phys. Rev. B 57, 10100–10109 (1998).
Acknowledgements
We thank A. Dongare and L. Zheiglei for providing us with an early version of the AEAM interatomic potential, and Alain Karma and Albert Davydov for helpful discussions. The computations were performed on stAMP supercomputing resources at Northeastern University. The study is supported by the National Science Foundation DMR CMMT Program (1106214). Part of this work was performed under the auspices of the US Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344 (L.A.Z.-R. and G.H.G.).
Author information
Authors and Affiliations
Contributions
H.W., L.A.Z.-R. and M.U. conceived the study. All authors were involved in development of the computational frameworks. H.W. and L.A.Z.-R. performed the computations. H.W. and M.U. performed the subsequent analyses. All authors discussed the results and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures, Notes, Methods and References
Supplementary Figures S1-S6, Supplementary Notes 1 and 2, Supplementary Methods and Supplementary References (PDF 726 kb)
Supplementary Movie 1
The growth dynamics at the droplet-nanowire interface for initial droplet composition XSi=0.48. The animation spans 450 configurations spaced 10 ps apart. The colour scheme is modified to better identify Si atoms nucleating on the truncating facets. Specifically, the atoms nucleating close to the contact line are shaded light blue and the intensity increases for atoms closer to the intersection of the main and the truncating facets. As before, atoms on the main facet are shaded dark blue. The modified scheme clearly shows that the circumferential growth all around the truncating facets is initiated at the contact line, away from the corner edges. (MOV 27841 kb)
Supplementary Movie 2
As for Supplementary Movie 1, but for XSi=0.463. The animation spans 350 configurations spaced 10 ps apart. (MOV 29171 kb)
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Wang, H., Zepeda-Ruiz, L., Gilmer, G. et al. Atomistics of vapour–liquid–solid nanowire growth. Nat Commun 4, 1956 (2013). https://doi.org/10.1038/ncomms2956
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms2956
This article is cited by
-
Growth and metal-decorated catalytic applications of silicon nanowires: a review
Bulletin of Materials Science (2023)
-
Phase diagrams of refractory bimetallic nanoalloys
Journal of Nanoparticle Research (2020)
-
Inducing imperfections in germanium nanowires
Nano Research (2017)
-
Molecular dynamics simulation studies of gold nano-cluster on silicon (001) surface
Indian Journal of Physics (2017)
-
Non-equilibrium induction of tin in germanium: towards direct bandgap Ge1−xSnx nanowires
Nature Communications (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.