Abstract
Integrin endocytic recycling is critical for cell migration, yet how recycled integrins assemble into new adhesions is unclear. By synchronizing endocytic disassembly of focal adhesions (FAs), we find that recycled integrins reassemble FAs coincident with their return to the cell surface and dependent on Rab5 and Rab11. Unexpectedly, endocytosed integrins remained in an active but unliganded state in endosomes. FAK and Src kinases co-localized with endocytosed integrin and were critical for FA reassembly by regulating integrin activation and recycling, respectively. FAK sustained the active integrin conformation by maintaining talin association with Rab11 endosomes in a type I phosphatidylinositol phosphate kinase (PIPKIγ)-dependent manner. In migrating cells, endocytosed integrins reassembled FAs polarized towards the leading edge, and this polarization required FAK. These studies identify unanticipated roles for FA proteins in maintaining endocytosed integrin in an active conformation. We propose that the conformational memory of endocytosed integrin enhances polarized reassembly of FAs to enable directional cell migration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ridley, A. J. et al. Cell migration: integrating signals from front to back. Science 302, 1704–1709 (2003).
Burridge, K., Chrzanowska-Wodnicka, M. & Zhong, C. Focal adhesion assembly. Trends Cell Biol. 7, 342–347 (1997).
Parsons, J. T., Horwitz, A. R. & Schwartz, M. A. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 11, 633–643 (2010).
Geiger, B., Spatz, J. P. & Bershadsky, A. D. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 10, 21–33 (2009).
Ross, T. D. et al. Integrins in mechanotransduction. Curr. Opin. Cell Biol. 25, 613–618 (2013).
Schiller, H. B. & Fassler, R. Mechanosensitivity and compositional dynamics of cell-matrix adhesions. EMBO Rep. 14, 509–519 (2013).
Smilenov, L. B., Mikhailov, A., Pelham, R. J., Marcantonio, E. E. & Gundersen, G. G. Focal adhesion motility revealed in stationary fibroblasts. Science 286, 1172–1174 (1999).
Mohl, C., Kirchgessner, N., Schafer, C., Hoffmann, B. & Merkel, R. Quantitative mapping of averaged focal adhesion dynamics in migrating cells by shape normalization. J. Cell Sci. 125, 155–165 (2012).
Stehbens, S. J. et al. CLASPs link focal-adhesion-associated microtubule capture to localized exocytosis and adhesion site turnover. Nat. Cell Biol. 16, 561–573 (2014).
Franco, S., Perrin, B. & Huttenlocher, A. Isoform specific function of calpain 2 in regulating membrane protrusion. Exp. Cell Res. 299, 179–187 (2004).
Franco, S. J. et al. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat. Cell Biol. 6, 977–983 (2004).
Crowley, E. & Horwitz, A. F. Tyrosine phosphorylation and cytoskeletal tension regulate the release of fibroblast adhesions. J. Cell Biol. 131, 525–537 (1995).
Chrzanowska-Wodnicka, M. & Burridge, K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 133, 1403–1415 (1996).
Vicente-Manzanares, M., Ma, X., Adelstein, R. S. & Horwitz, A. R. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 10, 778–790 (2009).
Ezratty, E. J., Bertaux, C., Marcantonio, E. E. & Gundersen, G. G. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J. Cell Biol. 187, 733–747 (2009).
Ezratty, E. J., Partridge, M. A. & Gundersen, G. G. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat. Cell Biol. 7, 581–590 (2005).
Chao, W. T. & Kunz, J. Focal adhesion disassembly requires clathrin-dependent endocytosis of integrins. FEBS Lett. 583, 1337–1343 (2009).
Wang, Y., Cao, H., Chen, J. & McNiven, M. A. A direct interaction between the large GTPase dynamin-2 and FAK regulates focal adhesion dynamics in response to active Src. Mol. Biol. Cell 22, 1529–1538 (2011).
Chao, W. T. et al. Type I phosphatidylinositol phosphate kinase beta regulates focal adhesion disassembly by promoting β1 integrin endocytosis. Mol. Cell Biol. 30, 4463–4479 (2010).
Bretscher, M. S. Circulating integrins: α5β1, α6β4 and Mac-1, but not α3β1, α4β1 or LFA-1. EMBO J. 11, 405–410 (1992).
Bretscher, M. S. Endocytosis and recycling of the fibronectin receptor in CHO cells. EMBO J. 8, 1341–1348 (1989).
Bretscher, M. S. & Aguado-Velasco, C. Membrane traffic during cell locomotion. Curr. Opin. Cell Biol. 10, 537–541 (1998).
Roberts, M. S., Woods, A. J., Dale, T. C., Van Der Sluijs, P. & Norman, J. C. Protein kinase B/Akt acts via glycogen synthase kinase 3 to regulate recycling of αvβ3 and α5β1 integrins. Mol. Cell Biol. 24, 1505–1515 (2004).
Powelka, A. M. et al. Stimulation-dependent recycling of integrin β1 regulated by ARF6 and Rab11. Traffic 5, 20–36 (2004).
Li, J. et al. Phosphorylation of ACAP1 by Akt regulates the stimulation-dependent recycling of integrin β1 to control cell migration. Dev. Cell 9, 663–673 (2005).
Pellinen, T. et al. Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of β1-integrins. J. Cell Biol. 173, 767–780 (2006).
Caswell, P. T. et al. Rab-coupling protein coordinates recycling of α5β1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J. Cell Biol. 183, 143–155 (2008).
Caswell, P. T. et al. Rab25 associates with α5β1 integrin to promote invasive migration in 3D microenvironments. Dev. Cell 13, 496–510 (2007).
Roberts, M., Barry, S., Woods, A., van der Sluijs, P. & Norman, J. PDGF-regulated rab4-dependent recycling of αvβ3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr. Biol. 11, 1392–1402 (2001).
White, D. P., Caswell, P. T. & Norman, J. C. αvβ3 and α5β1 integrin recycling pathways dictate downstream Rho kinase signaling to regulate persistent cell migration. J. Cell Biol. 177, 515–525 (2007).
Caswell, P. T., Vadrevu, S. & Norman, J. C. Integrins: masters and slaves of endocytic transport. Nat. Rev. Mol. Cell Biol. 10, 843–853 (2009).
Wu, X., Kodama, A. & Fuchs, E. ACF7 regulates cytoskeletal-focal adhesion dynamics and migration and has ATPase activity. Cell 135, 137–148 (2008).
Yeo, M. G. et al. Src SH2 arginine 175 is required for cell motility: specific focal adhesion kinase targeting and focal adhesion assembly function. Mol. Cell Biol. 26, 4399–4409 (2006).
Yue, J. et al. Microtubules regulate focal adhesion dynamics through MAP4K4. Dev. Cell 31, 572–585 (2014).
Slack-Davis, J. K. et al. Cellular characterization of a novel focal adhesion kinase inhibitor. J. Biol. Chem. 282, 14845–14852 (2007).
Hanke, J. H. et al. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 271, 695–701 (1996).
Kaplan, K. B., Swedlow, J. R., Varmus, H. E. & Morgan, D. O. Association of p60c-src with endosomal membranes in mammalian fibroblasts. J. Cell Biol. 118, 321–333 (1992).
Sandilands, E., Brunton, V. G. & Frame, M. C. The membrane targeting and spatial activation of Src, Yes and Fyn is influenced by palmitoylation and distinct RhoB/RhoD endosome requirements. J. Cell Sci. 120, 2555–2564 (2007).
Sandilands, E. et al. RhoB and actin polymerization coordinate Src activation with endosome-mediated delivery to the membrane. Dev. Cell 7, 855–869 (2004).
Gailit, J. & Ruoslahti, E. Regulation of the fibronectin receptor affinity by divalent cations. J. Biol. Chem. 263, 12927–12932 (1988).
Bouaouina, M., Lad, Y. & Calderwood, D. A. The N-terminal domains of talin cooperate with the phosphotyrosine binding-like domain to activate β1 and β3 integrins. J. Biol. Chem. 283, 6118–6125 (2008).
Byron, A. et al. Anti-integrin monoclonal antibodies. J. Cell Sci. 122, 4009–4011 (2009).
Kim, C., Ye, F. & Ginsberg, M. H. Regulation of integrin activation. Annu. Rev. Cell Dev. Biol. 27, 321–345 (2011).
Shattil, S. J., Kim, C. & Ginsberg, M. H. The final steps of integrin activation: the end game. Nat. Rev. Mol. Cell Biol. 11, 288–300 (2010).
Schill, N. J. & Anderson, R. A. Two novel phosphatidylinositol-4-phosphate 5-kinase type Igamma splice variants expressed in human cells display distinctive cellular targeting. Biochem. J. 422, 473–482 (2009).
Ling, K., Doughman, R. L., Firestone, A. J., Bunce, M. W. & Anderson, R. A. Type I gamma phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature 420, 89–93 (2002).
Ling, K. et al. Tyrosine phosphorylation of type Igamma phosphatidylinositol phosphate kinase by Src regulates an integrin-talin switch. J. Cell Biol. 163, 1339–1349 (2003).
Goksoy, E. et al. Structural basis for the autoinhibition of talin in regulating integrin activation. Mol. Cell 31, 124–133 (2008).
Martel, V. et al. Conformation, localization, and integrin binding of talin depend on its interaction with phosphoinositides. J. Biol. Chem. 276, 21217–21227 (2001).
Di Paolo, G. et al. Recruitment and regulation of phosphatidylinositol phosphate kinase type 1 gamma by the FERM domain of talin. Nature 420, 85–89 (2002).
Lawson, C. et al. FAK promotes recruitment of talin to nascent adhesions to control cell motility. J. Cell Biol. 196, 223–232 (2012).
Berginski, M. E., Vitriol, E. A., Hahn, K. M. & Gomez, S. M. High-resolution quantification of focal adhesion spatiotemporal dynamics in living cells. PLoS ONE 6, e22025 (2011).
Schober, M. et al. Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J. Cell Biol. 176, 667–680 (2007).
Michael, K. E., Dumbauld, D. W., Burns, K. L., Hanks, S. K. & Garcia, A. J. Focal adhesion kinase modulates cell adhesion strengthening via integrin activation. Mol. Biol. Cell 20, 2508–2519 (2009).
Alanko, J. et al. Integrin endosomal signalling suppresses anoikis. Nat. Cell Biol. 17, 1412–1421 (2015).
Belleudi, F., Scrofani, C., Torrisi, M. R. & Mancini, P. Polarized endocytosis of the keratinocyte growth factor receptor in migrating cells: role of SRC-signaling and cortactin. PLoS ONE 6, e29159 (2011).
Sandilands, E. et al. Src kinase modulates the activation, transport and signalling dynamics of fibroblast growth factor receptors. EMBO Rep. 8, 1162–1169 (2007).
Ilic, D. et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377, 539–544 (1995).
Sieg, D. J., Hauck, C. R. & Schlaepfer, D. D. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J. Cell Sci. 112 (Pt 16), 2677–2691 (1999).
Webb, D. J. et al. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 6, 154–161 (2004).
Lobert, V. H. et al. Ubiquitination of α5β1 integrin controls fibroblast migration through lysosomal degradation of fibronectin-integrin complexes. Dev. Cell 19, 148–159 (2010).
Takino, T. et al. Membrane type 1 matrix metalloproteinase regulates collagen-dependent mitogen-activated protein/extracellular signal-related kinase activation and cell migration. Cancer Res. 64, 1044–1049 (2004).
Brenner, K. A., Corbett, S. A. & Schwarzbauer, J. E. Regulation of fibronectin matrix assembly by activated Ras in transformed cells. Oncogene 19, 3156–3163 (2000).
Bouaouina, M., Harburger, D. S. & Calderwood, D. A. Talin and signaling through integrins. Methods Mol. Biol. 757, 325–347 (2012).
Ilic, D. et al. Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J. Cell Biol. 143, 547–560 (1998).
Sonoda, Y. et al. Anti-apoptotic role of focal adhesion kinase (FAK). Induction of inhibitor-of-apoptosis proteins and apoptosis suppression by the overexpression of FAK in a human leukemic cell line, HL-60. J. Biol. Chem. 275, 16309–16315 (2000).
Laukaitis, C. M., Webb, D. J., Donais, K. & Horwitz, A. F. Differential dynamics of α5 integrin, paxillin, and α-actinin during formation and disassembly of adhesions in migrating cells. J. Cell Biol. 153, 1427–1440 (2001).
Junutula, J. R. et al. Molecular characterization of Rab11 interactions with members of the family of Rab11-interacting proteins. J. Biol. Chem. 279, 33430–33437 (2004).
Gomes, E. R. & Gundersen, G. G. Real-time centrosome reorientation during fibroblast migration. Methods Enzymol. 406, 579–592 (2006).
Acknowledgements
We thank G. DiPaolo and E. Marcantonio for suggestions and R. Horwitz (Allen Institute for Cell Science, USA), J. Goldenring (Vanderbilt University, USA), D. Calderwood (Yale University, USA), D. Schlaepfer (University of California, San Diego, USA) and R. Anderson (University of Wisconsin-Madison, USA) for reagents. This work was supported by NIH grants GM062939 and GM099481 to G.G.G.
Author information
Authors and Affiliations
Contributions
G.P.F.N. conducted and designed experiments, analysed and interpreted the data and wrote the manuscript. E.J.E. conducted initial experiments, interpreted the data and edited the manuscript. G.G.G. designed the experiments, analysed and interpreted the data and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
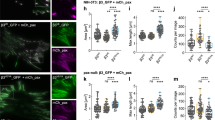
Supplementary Figure 1 FAs reassembled after NZ washout contain typical FA markers and are biased towards the cell periphery.
(a) Immunofluorescence images of FAK-pY397, paxillin and phalloidin staining (actin) of NIH3T3 fibroblasts after NZ treatment and washout for the indicated times. Bar, 10 μm. (b) Quantification of cell area before and after MT-induced FA disassembly. n = 25 cells pooled from 3 independent experiments. Box and whisker plots show median values by horizontal lines, 75% of values by boxes and 95% of the range by whiskers. (c, left panels) Immunofluorescence of paxillin-EGFP NIH3T3 fibroblast cell line showing a similar distribution of paxillin-EGFP and endogenous FAK-pY397. Bar, 15 μm. (c, right panels) Immunofluorescence of NIH3T3 fibroblasts stained for paxillin and vinculin showing that FAs are similar in size, number and distribution compared to the FAs in paxillin-EGFP NIH3T3 fibroblasts. Bar, 15 μm. (d) Representative immunoblots of paxillin levels in paxillin-EGFP NIH3T3 fibroblasts; 2 independent experiments. Note that paxillin-EGFP is expressed at levels comparable to endogenous paxillin. (e) Representative immunoblots of paxillin-pY118 and total paxillin in NIH3T3 fibroblast lysates after NZ washout in the presence of DMSO, 10 μM PF228 or 10 μM PP2. Tubulin, loading control; data represent 1 out of 2 independent experiments. (f) Quantification of cell migration velocity for parental NIH3T3 fibroblasts and paxillin-EGFP NIH3T3 fibroblasts. DIC images were taken at 5 min intervals for 12 h; n = 35 cells pooled from 3 independent experiments. (g) TIRF images from a movie of paxillin-EGFP NIH3T3 fibroblasts at 0, 60 and 100 min after NZ washout. Bar, 5 μm. (h) FA reassembly occurs preferentially at the cell periphery. Left: diagram illustrating the regions used to quantify the distribution of reassembled FAs: FAs located within 2 μm of the cell periphery were classified as peripheral; those outside the 2 μm range were classified as internal. Middle and right: quantification of peripheral FA area and number as defined in the diagram; n = 10 cells pooled from 2 independent experiments. Error bars (f,h) are mean ± SD. Student’s two-tailed unpaired t-test; P values are indicated; ns, not significant. See Supplementary Figure 9 for uncropped images of blots.
Supplementary Figure 2 FA reassembly involves endocytic recycling.
(a) Recycling of biotinylated integrin following FA disassembly. NIH3T3 fibroblasts were treated with NZ for 3 h and then surface labeled with biotin at 4 °C. NZ was washed out and biotinylated proteins were allowed to internalize for 60 min. Biotin was removed from receptors remaining at the cell surface by reduction with MesNa at 4 °C. Cells were then rewarmed to 37 °C for the times indicated followed by a second reduction. Cells were lysed and integrin-biotinylation was determined by capture-ELISA using microtiter wells coated with α5 integrin antibody; data represent the mean of n = 6 independent experiments. [ok?](b) Representative immunoblots of α5 integrin in NIH3T3 fibroblasts lysates after NZ washout. Vinculin, loading control; data represent 1 out of 3 independent experiments. (c) Immunofluorescence images of α5 integrin-GFP, Rab11 and GM-130 (Golgi) in NIH3T3 fibroblasts expressing α5 integrin-GFP and fixed at 60 min of NZ washout. Arrows indicate the Rab11 ERC; arrowhead indicates the Golgi. Note colocalization of α5 integrin-GFP with the Rab11 ERC, but not the Golgi. Bar, 5 μm. (d) Immunofluorescence images of paxillin-pY118, GM-130 (Golgi) and MTs in NIH3T3 fibroblasts after NZ washout for the indicated times in the presence of either 4 μg/ml brefeldin A or 0.1% ethanol (vehicle). Bar, 15 μm. (e) Immunofluorescence images of vinculin and Rab11 in NIH3T3 fibroblasts expressing Rab11 WT-EGFP or Rab11 S25N-EGFP fixed at 120 min of NZ washout. Arrows indicate transfected cells. Note reassembly of FAs in cells expressing Rab11 WT but not dominant negative Rab11 S25N. Bar, 20 μm. (f) Immunofluorescence image of zyxin and phalloidin actin staining of a Rab5 S34N-EGFP-transfected NIH3T3 fibroblast (arrow) 120 min after NZ washout. Bar, 15 μm. (g) Quantification of FA disassembly and reassembly in NIH3T3 fibroblasts transfected with the indicated EGFP-tagged Rab constructs; n = 3 independent experiments; 150 cells analysed per condition in each experiment. (h) Representative immunoblots of Rab11a/b in NIH3T3 fibroblasts treated with non-coding (NC) or Rab11a/b siRNAs. Tubulin, loading control. Data represent 1 out of 3 independent experiments. Error bars are (a) SEM and (g) mean ± SD. Student’s two-tailed unpaired t-test; P values are indicated. See Supplementary Figure 9 for uncropped images of blots.
Supplementary Figure 3 FAK and SFK inhibitors inhibit FA reassembly and do so in a reversible fashion.
(a) Immunofluorescence images of FAK, vinculin and MTs in NIH3T3 fibroblasts at the indicated times after NZ washout in the presence of vehicle (DMSO) or PF228 (1 or 5 μM). Note that even low concentrations of PF228 still inhibit focal adhesion reassembly without affecting focal adhesion disassembly. Bar, 10 μm. (b) Immunofluorescence images of vinculin, paxillin and MTs in NIH3T3 fibroblasts at indicated times after NZ washout in the presence of DMSO or 2 μM FAK inhibitor-I. Note that FAK inhibitor-I does not affect FA disassembly (60 min NZ washout) but prevents reassembly of FAs (120 min NZ washout). Bar, 15 μm. (c) Immunofluorescence images of vinculin and MTs in NIH3T3 after NZ washout in the presence of DMSO or 10 μM PF228 or 10 μM PP2. After 120 min of NZ washout, kinase inhibitors were washed out and cells were fixed at the indicated times of “release”. Bar, 15 μm (d) Quantification of FA reassembly following drug release of cells treated as in “c”; n = 3 independent experiments; 200 cells analysed per condition in each experiment. Error bars are mean ± SD; ns, not significant. (e) Immunofluorescence images of src and Rab11-EGFP in NIH3T3 fibroblasts expressing Rab11-EGFP fixed at 60 min after NZ washout. Arrows indicate src colocalizing with Rab11 in the ERC. Bar, 15 μm.
Supplementary Figure 4 FA reassembly is rescued by Mn2+ in FAK−/− cells transfected with FAK kinase mutants but not in Rab11-depleted cells.
(a) Immunofluorescence images of vinculin in NIH3T3 fibroblasts treated with non-coding (NC) or Rab11a/b siRNAs and fixed at 120 min after NZ washout. MnCl2 (100 μM) was added 90 min after NZ washout. Bar, 15 μm. (b) Quantification of FA reassembly of cells treated as in “a”; n = 3 independent experiments; 150 cells analysed per condition in each experiment. (c) Immunofluorescence images of FAK (WT or different mutants) and vinculin in FAK-/- cells expressing FAK-WT-GFP, FAK-Y397F-GFP or FAK-K454R-HA and fixed at the indicated times after NZ washout. MnCl2 (100 μM) was added to the cells 60 min after NZ washout. Arrows indicate transfected cells. Bar, 15 μm. (d) Quantification of FA disassembly and reassembly in transfected FAK-/- cells at the indicated times after NZ washout as in “c”; n = 3 independent experiments; 100 cells analysed per condition in each experiment. Error bars are mean ± SD. Student’s two-tailed unpaired t-test; P values are indicated; ns, not significant.
Supplementary Figure 5 FAK inhibitor blocks FA reassembly without interfering with FA disassembly in HT1080 fibrosarcoma cells.
(a) Immunofluorescence images of vinculin, paxillin-pY118 and MTs in HT1080 fibrosarcoma cells fixed at the indicated times after NZ washout in the presence of DMSO or 10 μM PF228. Bar, 25 μm. (b) Quantification of FA disassembly (60 min NZ washout) and reassembly (120 min NZ washout) in HT1080 cells treated with DMSO or PF228 as in “a”. n = 3 independent experiments, 200 cells analysed per condition in each experiment. Bars are SD. (c) Immunofluorescence images of active β1 integrin (12G10 antibody) and FAK-pY397 in HT1080 cells fixed 60 min after NZ washout. Arrows indicate colocalization of active integrin and FAK-pY397 signals. Bar, 10 μm. (d) Immunofluorescence images of FAK-pY397 in HT1080 cells fixed 60 min after NZ washout in the presence of DMSO or 80 μM dynasore. Bar, 15 μm. (e) Immunofluorescence images of FAK-pY397 and Rab11 in HT1080 cells fixed 60 min after NZ washout in the presence of DMSO or 80 μM dynasore. Bar, 15 μm. (f) Quantification of FA disassembly in HT1080 cells fixed 60 min after NZ washout in the presence of DMSO or 80 μM dynasore; n = 60 cells analysed per condition, pooled from 2 independent experiments. Box and whisker plots represent values as defined in Supplementary Fig. 1b legend. Student’s two-tailed unpaired t-test; P values are indicated; ns, not significant.
Supplementary Figure 6 An antibody to a second epitope on active β1 integrin detects endocytosed integrin in HT1080 fibrosarcoma cells and SFKs are not critical for maintaining integrin activation in the ERC.
(a) Immunofluorescence images of active β1 integrin (HUTS-4 antibody), total β1 integrin (K20 antibody) and Rab11 in HT1080 cells fixed at 60 min after NZ washout. Boxed regions are enlarged in the images on the right. Bar, 10 μm. (b) Immunofluorescence images of active β1 integrin (12G10 antibody), total β1 integrin (K20 antibody) and Rab11 in HT1080 cells fixed at 60 min after NZ washout in the presence of DMSO or 10 μM PP2. Boxed regions are enlarged in the images on the right. Bar, 15 μm. (c) Quantification of fluorescence intensity of active β1 integrin measured in the Rab11 ERC of HT1080 cells 60 min after NZ washout in DMSO or 10 μM PP2. n = 3 independent experiments, 30 cells analysed per condition in each experiment. Box and whisker plots represent values as defined in Supplementary Fig. 1b legend; ns, not significant.
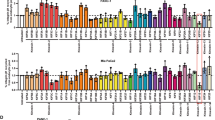
Supplementary Figure 7 Talin is required for FA reassembly in NIH3T3 fibroblasts and localization of PIPKIγi1 in HT1080 cells.
(a) Representative immunoblots of lysates from NIH3T3 fibroblasts treated with non-coding (NC) or different talin1 siRNAs. Tubulin is a loading control. Data represent 1 out or 3 independent experiments. (b) Immunofluorescence images of paxillin-pY31 and MTs in NIH3T3 fibroblasts transfected with NC or talin1 siRNA and fixed at the indicated times after NZ washout. Bar, 15 μm. (c) Quantification of FA disassembly and reassembly after NZ washout from NIH3T3 fibroblasts treated with non-coding (NC) or talin siRNAs. n = 4 independent experiments, 150 cells analysed per condition in each experiment. Error bars are mean ± SD. (d) Immunofluorescence images of talin and Rab11 in NIH3T3 fibroblasts 60 min after NZ washout. Bar, 15 μm. (e,f) Immunofluorescence images of PIPKIγi1-GFP and vinculin in HT1080 cells before (e) and after (f) NZ washout. Cells were transduced with PIPKIγi1-GFP using retrovirus. Note that PIPKIγi1 does not localize to FAs before NZ washout or to the Rab11 ERC after NZ washout. Bar, 20 μm. Student’s two-tailed unpaired t-test P values are provided. See Supplementary Figure 9 for uncropped images of blots.
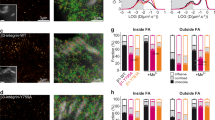
Supplementary Figure 8 PIPKIγ is required for FA reassembly and migrating cells accumulate active integrin in the Rab11 ERC in a FAK-dependent manner.
(a) Immunofluorescence images of paxillin-pY31 and MTs in HT1080 cells treated with a noncoding (NC) or a smart pool mixture of PIPKIγ siRNAs and fixed at the indicated times after NZ washout. Bar, 15 μm. (b) Quantification of FA disassembly and reassembly after NZ washout in HT1080 cells. n = 5 independent experiments; 150 cells analysed per condition in each experiment. (c) Representative immunoblots of lysates from HT1080 cells treated with a noncoding (NC) or a smart pool mixture of PIPKIγ siRNAs. Tubulin, loading control. Data represent 1 out of 3 independent experiments. (d) Immunofluorescence images of paxillin in NIH3T3 fibroblasts seeded on FN-coated cover slips for 4 h at low confluency and then allowed to migrate after treatment with vehicle (DMSO) or FAK inhibitor (PF228) for the indicated times. Two examples for each condition are shown. Note reduced FAs and lack of new focal adhesion in cells treated with PF228. Bar, 15 μm. (e) Immunofluorescence images of active β1 integrin, total β1 integrin and Rab11 in wounded monolayers of HT1080 cells treated with SFKs inhibitor alone (PP2 2h) or treated with PP2 for 2 h followed by addition of FAK inhibitor for another 2 h (PP2 2h + PF228 2h). Arrows point to active β1 integrin accumulation in the Rab11 ERC upon treatment with PP2. Note lack of active integrin staining in the Rab11 ERC after treatment with both PP2 and PF228. Bar, 20 μm. (f) Quantification of active β1 integrin signal (top) or total β1 integrin signal (bottom) in the Rab11 ERC of cells located at wounded monolayers; n = 60 cells analysed per condition, pooled from 2 independent experiments,. Box and whisker plots (f) represent values as defined in Supplementary Fig. 1b legend. Error bars (b) are mean ± SD. Student’s two-tailed unpaired t-test; P values are indicated; ns, not significant. See Supplementary Figure 9 for uncropped images of blots.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2022 kb)
Time-lapse movie showing FA disassembly and reassembly.
NIH3T3 fibroblasts stably expressing paxillin-EGFP were imaged by TIRF microscopy after NZ treatment for 3 h followed by NZ washout to stimulate FA disassembly and reassembly. Images were captured every 40 sec. (AVI 62450 kb)
Rights and permissions
About this article
Cite this article
Nader, G., Ezratty, E. & Gundersen, G. FAK, talin and PIPKIγ regulate endocytosed integrin activation to polarize focal adhesion assembly. Nat Cell Biol 18, 491–503 (2016). https://doi.org/10.1038/ncb3333
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3333
This article is cited by
-
Focal adhesion kinase: from biological functions to therapeutic strategies
Experimental Hematology & Oncology (2023)
-
Endocytosis in cancer and cancer therapy
Nature Reviews Cancer (2023)
-
Organization, dynamics and mechanoregulation of integrin-mediated cell–ECM adhesions
Nature Reviews Molecular Cell Biology (2023)
-
Wun2-mediated integrin recycling promotes apoptotic cell clearance in Drosophila melanogaster
Cell Death & Differentiation (2022)
-
Dynamic regulation of KIF15 phosphorylation and acetylation promotes focal adhesions disassembly in pancreatic cancer
Cell Death & Disease (2022)