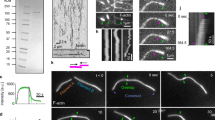
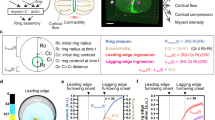
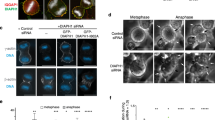
Abstract
Animal cell cytokinesis requires a contractile ring of crosslinked actin filaments and myosin motors. How contractile rings form and are stabilized in dividing cells remains unclear. We address this problem by focusing on septins, highly conserved proteins in eukaryotes whose precise contribution to cytokinesis remains elusive. We use the cleavage of the Drosophila melanogaster embryo as a model system, where contractile actin rings drive constriction of invaginating membranes to produce an epithelium in a manner akin to cell division. In vivo functional studies show that septins are required for generating curved and tightly packed actin filament networks. In vitro reconstitution assays show that septins alone bundle actin filaments into rings, accounting for the defects in actin ring formation in septin mutants. The bundling and bending activities are conserved for human septins, and highlight unique functions of septins in the organization of contractile actomyosin rings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Eggert, U. S., Mitchison, T. J. & Field, C. M. Animal cytokinesis: from parts list to mechanisms. Ann. Rev. Biochem. 75, 543–566 (2006).
Green, R. A., Paluch, E. & Oegema, K. Cytokinesis in animal cells. Ann. Rev. Cell Dev. Biol. 28, 29–58 (2012).
Murrell, M. P. & Gardel, M. L. F-actin buckling coordinates contractility and severing in a biomimetic actomyosin cortex. Proc. Natl Acad. Sci. USA 109, 20820–20825 (2012).
Vogel, S. K., Petrasek, Z., Heinemann, F. & Schwille, P. Myosin motors fragment and compact membrane-bound actin filaments. eLife 2, e00116 (2013).
Glotzer, M. The molecular requirements for cytokinesis. Science 307, 1735–1739 (2005).
Saarikangas, J. & Barral, Y. The emerging functions of septins in metazoans. EMBO Rep. 12, 1118–1126 (2011).
Bertin, A. et al. Phosphatidylinositol-4,5-bisphosphate promotes budding yeast septin filament assembly and organization. J. Mol. Biol. 404, 711–731 (2010).
Zhang, J. et al. Phosphatidylinositol polyphosphate binding to the mammalian septin H5 is modulated by GTP. Curr. Biol. 9, 1458–1467 (1999).
Field, C. M., Coughlin, M., Doberstein, S., Marty, T. & Sullivan, W. Characterization of anillin mutants reveals essential roles in septin localization and plasma membrane integrity. Development 132, 2849–2860 (2005).
Kinoshita, M., Field, C. M., Coughlin, M. L., Straight, A. F. & Mitchison, T. J. Self- and actin-templated assembly of mammalian septins. Dev. Cell 3, 791–802 (2002).
Oegema, K., Savoian, M. S., Mitchison, T. J. & Field, C. M. Functional analysis of a human homologue of the Drosophila actin binding protein anillin suggests a role in cytokinesis. J. Cell Biol. 150, 539–552 (2000).
Joo, E., Surka, M. C. & Trimble, W. S. Mammalian SEPT2 is required for scaffolding nonmuscle myosin II and its kinases. Dev. Cell 13, 677–690 (2007).
Mostowy, S. et al. Entrapment of intracytosolic bacteria by septin cage-like structures. Cell Host Microb. 8, 433–444 (2010).
Schmidt, K. & Nichols, B. J. A barrier to lateral diffusion in the cleavage furrow of dividing mammalian cells. Curr. Biol. 14, 1002–1006 (2004).
Caudron, F. & Barral, Y. Septins and the lateral compartmentalization of eukaryotic membranes. Dev. Cell 16, 493–506 (2009).
Liu, J., Fairn, G. D., Ceccarelli, D. F., Sicheri, F. & Wilde, A. Cleavage furrow organization requires PIP(2)-mediated recruitment of anillin. Curr. Biol. 22, 64–69 (2012).
Kechad, A., Jananji, S., Ruella, Y. & Hickson, G. R. Anillin acts as a bifunctional linker coordinating midbody ring biogenesis during cytokinesis. Curr. Biol. 22, 197–203 (2012).
Adam, J. C., Pringle, J. R. & Peifer, M. Evidence for functional differentiation among Drosophila septins in cytokinesis and cellularization. Mol. Biol. Cell 11, 3123–3135 (2000).
Field, C. M. & Alberts, B. M. Anillin, a contractile ring protein that cycles from the nucleus to the cell cortex. J. Cell Biol. 131, 165–178 (1995).
Lecuit, T. & Wieschaus, E. Polarized insertion of new membrane from a cytoplasmic reservoir during cleavage of the Drosophila embryo. J. Cell Biol. 150, 849–860 (2000).
Schejter, E. D. & Wieschaus, E. bottleneck acts as a regulator of the microfilament network governing cellularization of the Drosophila embryo. Cell 75, 373–385 (1993).
Thomas, J. H. & Wieschaus, E. src64 and tec29 are required for microfilament contraction during Drosophila cellularization. Development 131, 863–871 (2004).
Neufeld, T. P. & Rubin, G. M. The Drosophila peanut gene is required for cytokinesis and encodes a protein similar to yeast putative bud neck filament proteins. Cell 77, 371–379 (1994).
Field, C. M. et al. A purified Drosophila septin complex forms filaments and exhibits GTPase activity. J. Cell Biol. 133, 605–616 (1996).
Royou, A., Field, C., Sisson, J. C., Sullivan, W. & Karess, R. Reassessing the role and dynamics of nonmuscle myosin II during furrow formation in early Drosophila embryos. Mol. Biol. Cell 15, 838–850 (2004).
Fullilove, S. L. & Jacobson, A. G. Nuclear elongation and cytokinesis in Drosophila montana. Dev. Biol. 26, 560–577 (1971).
Carvalho, A., Desai, A. & Oegema, K. Structural memory in the contractile ring makes the duration of cytokinesis independent of cell size. Cell 137, 926–937 (2009).
Padash Barmchi, M., Rogers, S. & Hacker, U. DRhoGEF2 regulates actin organization and contractility in the Drosophila blastoderm embryo. J. Cell Biol. 168, 575–585 (2005).
Grosshans, J. et al. RhoGEF2 and the formin Dia control the formation of the furrow canal by directed actin assembly during Drosophila cellularisation. Development 132, 1009–1020 (2005).
Wenzl, C., Yan, S., Laupsien, P. & Grosshans, J. Localization of RhoGEF2 during Drosophila cellularization is developmentally controlled by Slam. Mech. Dev. 127, 371–384 (2010).
Zhang, L. & Ward, R. E. Distinct tissue distributions and subcellular localizations of differently phosphorylated forms of the myosin regulatory light chain in Drosophila. Gene Expression Patterns 11, 93–104 (2011).
Kress, A. et al. Mapping the local organization of cell membranes using excitation-polarization-resolved confocal fluorescence microscopy. Biophys. J. 105, 127–136 (2013).
Sirajuddin, M. et al. Structural insight into filament formation by mammalian septins. Nature 449, 311–315 (2007).
Garcia, G. 3rd. et al. Subunit-dependent modulation of septin assembly: budding yeast septin Shs1 promotes ring and gauze formation. J. Cell Biol. 195, 993–1004 (2011).
Farkasovsky, M., Herter, P., Voss, B. & Wittinghofer, A. Nucleotide binding and filament assembly of recombinant yeast septin complexes. Biol. Chem. 386, 643–656 (2005).
Weirich, C. S., Erzberger, J. P. & Barral, Y. The septin family of GTPases: architecture and dynamics. Nat. Rev. 9, 478–489 (2008).
Holmes, K. C. Structural biology: actin in a twist. Nature 457, 389–390 (2009).
Jansen, S. et al. Mechanism of actin filament bundling by fascin. J. Biol. Chem. 286, 30087–30096 (2011).
Faix, J. et al. Cortexillins, major determinants of cell shape and size, are actin-bundling proteins with a parallel coiled-coil tail. Cell 86, 631–642 (1996).
Bertin, A. et al. Saccharomyces cerevisiae septins: supramolecular organization of heterooligomers and the mechanism of filament assembly. Proc. Natl Acad. Sci. USA 105, 8274–8279 (2008).
Frazier, J. A. et al. Polymerization of purified yeast septins: evidence that organized filament arrays may not be required for septin function. J. Cell Biol. 143, 737–749 (1998).
Hickson, G. R. & O’Farrell, P. H. Rho-dependent control of anillin behavior during cytokinesis. J. Cell Biol. 180, 285–294 (2008).
D’Avino, P. P. et al. Interaction between Anillin and RacGAP50C connects the actomyosin contractile ring with spindle microtubules at the cell division site. J. Cell Sci. 121, 1151–1158 (2008).
Estey, M. P., Di Ciano-Oliveira, C., Froese, C. D., Bejide, M. T. & Trimble, W. S. Distinct roles of septins in cytokinesis: SEPT9 mediates midbody abscission. J. Cell Biol. 191, 741–749 (2010).
Gilden, J. K., Peck, S., Chen, Y. C. & Krummel, M. F. The septin cytoskeleton facilitates membrane retraction during motility and blebbing. J. Cell Biol. 196, 103–114 (2012).
Sedzinski, J. et al. Polar actomyosin contractility destabilizes the position of the cytokinetic furrow. Nature 476, 462–466 (2011).
Guillot, C. & Lecuit, T. Adhesion disengagement uncouples intrinsic and extrinsic forces to drive cytokinesis in epithelial tissues. Dev. Cell 24, 227–241 (2013).
Founounou, N., Loyer, N. & Le Borgne, R. Septins regulate the contractility of the actomyosin ring to enable adherens junction remodelling during cytokinesis of epithelial cells. Dev. Cell 24, 242–255 (2013).
Mostowy, S. et al. A role for septins in the interaction between the Listeria monocytogenes INVASION PROTEIN InlB and the Met receptor. Biophys. J. 100, 1949–1959 (2011).
Tooley, A. J. et al. Amoeboid T lymphocytes require the septin cytoskeleton for cortical integrity and persistent motility. Nat. Cell Biol. 11, 17–26 (2009).
Dagdas, Y. F. et al. Septin-mediated plant cell invasion by the rice blast fungus, Magnaporthe oryzae. Science 336, 1590–1595 (2012).
Pan, F., Malmberg, R. L. & Momany, M. Analysis of septins across kingdoms reveals orthology and new motifs. BMC Evol. Biol. 7, 103 (2007).
Sellin, M. E., Sandblad, L., Stenmark, S. & Gullberg, M. Deciphering the rules governing assembly order of mammalian septin complexes. Mol. Biol. Cell 22, 3152–3164 (2011).
Kim, M. S., Froese, C. D., Estey, M. P. & Trimble, W. S. SEPT9 occupies the terminal positions in septin octamers and mediates polymerization-dependent functions in abscission. J. Cell Biol. 195, 815–826 (2011).
Gittes, F., Mickey, B., Nettleton, J. & Howard, J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J. Cell Biol. 120, 923–934 (1993).
Tang, J. X., Kas, J. A., Shah, J. V. & Janmey, P. A. Counterion-induced actin ring formation. Eur. Biophys. J. 30, 477–484 (2001).
Taylor, K. A., Taylor, D. W. & Schachat, F. Isoforms of α-actinin from cardiac, smooth, and skeletal muscle form polar arrays of actin filaments. J. Cell Biol. 149, 635–646 (2000).
Surka, M. C., Tsang, C. W. & Trimble, W. S. The mammalian septin MSF localizes with microtubules and is required for completion of cytokinesis. Mol. Biol. Cell 13, 3532–3545 (2002).
Kinoshita, M. et al. Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev. 11, 1535–1547 (1997).
Mostowy, S. & Cossart, P. Septins: the fourth component of the cytoskeleton. Nature reviews. Mol. Cell Biol. 13, 183–194 (2012).
Lecuit, T. & Pilot, F. Developmental control of cell morphogenesis: a focus on membrane growth. Nat. Cell Biol. 5, 103–108 (2003).
Murthy, M., Teodoro, R. O., Miller, T. P. & Schwarz, T. L. Sec5, a member of the exocyst complex, mediates Drosophila embryo cellularization. Development 137, 2773–2783 (2010).
Mavrakis, M., Rikhy, R. & Lippincott-Schwartz, J. Plasma membrane polarity and compartmentalization are established before cellularization in the fly embryo. Dev. Cell 16, 93–104 (2009).
Rauzi, M., Lenne, P. F. & Lecuit, T. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature 468, 1110–1114 (2010).
Mavrakis, M., Rikhy, R., Lilly, M. & Lippincott-Schwartz, J. in Current Protocols in Cell Biology (eds Bonifacino, J.S. et al.) (ed Bonifacino, J.S.et al.) Ch. 4, Unit 4.18 (John Wiley, (2008).
Levayer, R., Pelissier-Monier, A. & Lecuit, T. Spatial regulation of Dia and Myosin-II by RhoGEF2 controls initiation of E-cadherin endocytosis during epithelial morphogenesis. Nat. Cell Biol. 13, 529–540 (2011).
Diebold, M. L., Fribourg, S., Koch, M., Metzger, T. & Romier, C. Deciphering correct strategies for multiprotein complex assembly by co-expression: application to complexes as large as the histone octamer. J. Struct. Biol. 175, 178–188 (2011).
Pardee, J. D. & Spudich, J. A. Purification of muscle actin. Methods Enzymol. 85 Pt B, 164–181 (1982).
Pollard, T. D. A guide to simple and informative binding assays. Mol. Biol. Cell 21, 4061–4067 (2010).
Gentry, B. S. et al. Multiple actin binding domains of Ena/VASP proteins determine actin network stiffening. Eur. Biophys. J. 41, 979–990 (2012).
Acknowledgements
We thank the Lecuit and Lenne groups for discussions and for providing a fruitful environment during the course of this work. We thank J-P. Chauvin, A. Aouane and F. Richard at the IBDM electron microscopy facility for help with embryo processing and image acquisition, G. Pehau-Arnaudet at Imagopole (Institut Pasteur) and the electron microscopy facility at Imagif (Gif sur Yvette) for technical help with imaging of in vitro actin–septin assemblies, P. Ferrand, X. Wang, J. Duboisset and H. Rigneault (Institut Fresnel) for their contribution in the implementation of polarization-resolved fluorescence microscopy, C. Romier and C. Fernández-Tornero for discussions on septin purification, F. Maina for key advice on phosphoprotein westerns, M. Kuit-Vinkenoog for G-actin purification, and A. Kamor and C. Chandre for Matlab and POV-ray code for drawing FC tori. M.M., Y.A-G. and F.I. were supported by the ANR Blanc ARCHIPLAST (T.L.), the Fondation pour la Recherche Medicale (équipe labelisée, T.L.), the Association pour la Recherche contre la Cancer (Programme ARC, SL220120605305) and the CNRS. M.M., A.B., F-C.T., J.A. and G.H.K. were supported by two PHC Van Gogh grants (no. 25005UA and no. 28879SJ, ministères des Affaires étrangères et de l’Enseignement supérieur et de la Recherche), and G.H.K., F-C.T. and J.A. were supported by a VIDI grant from NWO and by the Foundation for Fundamental Research on Matter (FOM). A.K. and S.B. were supported by the CNRS, the ANR BLANC grants 150902 (ReceptORIENT) and 18818 (RADORDER) and the region Provence Alpes Côte d’Azur. This work was supported by the national infrastructure France Bio-Imaging and the Nikon Application Center Marseille.
Author information
Authors and Affiliations
Contributions
Experiments were conceived and planned by M.M., G.H.K. and T.L. Experiments were performed by M.M., Y.A-G., F-C.T., J.A., A.B., F.I. and A.K. Data analysis was performed by M.M., Y.A-G., A.B., A.K. and S.B. M.M. wrote the first version of the manuscript, and T.L. and G.H.K. contributed to the writing of the final version. F-C.T., J.A., A.B. and S.B. contributed to the writing of the methods. All authors participated in the discussion of the data and in producing the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 FC membrane polarity is not affected during cellularization of septin mutants.
a, b, Confocal images of DSep1 (a, left) and DSep2 (b, left) in a top view of the FC in a wild-type embryo. Side views of the FC in wild-type and septin mutant depicting the localization of the lateral membrane marker, Scb, and DSep1 (a, right) and Scb and DSep2 (b, right). (c) Confocal images of the lateral membrane marker, Dlg, and the FC membrane markers, DPatj, Slam and DRhoGEF2, in side views of the FC during the fast phase of wild-type and septin mutant embryos. Notice the flat morphology of FC membranes in mutants (arrowheads). d, Confocal images of DPatj and Dlg in a top view of the FC during the fast phase of wild-type and septin mutant embryos. A higher magnification of a FC in the mutant (dashed box) shows the segregation of Dlg and DPatj. e, Localization of myosin heavy chain (MHC), Pnut/hSep7 and DSep1 at the FC of septin mutant embryos. A higher magnification of the FC region is depicted on the right. f, Two-photon images of UtrCHD::GFP during cellularization of wild-type (top panel) and septin mutant (bottom panel). Scale bars, 5 μm.
Supplementary Figure 2 Defects in F-actin organization during cellularization of septin mutants.
a, c, d, Confocal images of Actin (phalloidin-stained) during the early slow phase (A, 1-3 depict three representative examples), the early fast phase (C, 1-2 depict two representative examples) and the late fast phase (D, right) of septin mutants. Actin in wild type embryos of the respective stages are shown in c and d, left for comparison. (b) Side view of FCs in a septin mutant stained for Actin, MHC and Pnut/hSep7. e, Quantification of FC perimeters from segmentation analysis of wild-type (N=32–144 FCs/timepoint/embryo) embryos. All the N numbers of FCs per timepoint per embryo are provided in Supplementary Table 1. Constriction rates are calculated from linear regression analysis in the regions depicted by the two black line segments. Error bars are mean ± s.d. f, Definition of circularity. Scale bars, 5 μm.
Supplementary Figure 3 Electron microscopy of FCs in wild-type and septin mutants.
(a) Electron micrograph of a FC section depicting how measurements of FC coat densities (quantified in Fig. 4g) were performed. I, Mean pixel intensity. (b) Examples of FC sections in wild-type and septin mutants at low magnification. (c) Electron micrograph of grazing sections through the side of a FC in a wild-type (left) and a septin mutant (right). The schematic depicts the grazing sections with respect to the FC geometry. Dashed black lines follow the FC membrane contour. White guidelines in wild-type are oriented parallel to the observed arrays of filaments. d, Electron micrograph of a top view of a wild-type FC depicting the curved electron-dense coat (white brackets) at the membrane e, Electron micrograph of a grazing section through the base of a wild-type FC. White guidelines are oriented parallel to the observed arrays of filaments.
Supplementary Figure 4 In vitro reconstituted actin filaments are bundled in the presence of septins.
a, b SDS-PAGE analysis (Coomassie blue stained) of fractions from low speed co-sedimentation of 1 μM F-actin co-polymerized with the indicated concentrations of Fascin (a) or Drosophila septins (b). T, total. S, supernatant. P, pellet.
Supplementary Figure 5 Characterization of DSep1 and DSep2 antibodies generated in this study.
a, b, Homology models of DSep1 (a) and DSep2 (b) structures depicting in red the amino acid (aa) sequence and respective regions in the structure that were used as epitopes for antibody production. c, d, Western blot of purified recombinant septin complexes (c) and wild-type embryo lysates (d) using the DSep1 and DSep2 antibodies generated in this study. e, SDS-PAGE analysis of recombinant septin complexes of His6-hSep2, hSep6 and hSep7-Strep.
Supplementary Figure 6 Representative entire images of immunoblotting and SDS-PAGE.
Boxed areas were cropped for designated figures. Dotted lines denote separations of membranes for dual labeling.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1032 kb)
Supplementary Table 1
Supplementary Information (XLSX 40 kb)
Side view of membrane invagination during cellularization in a MRLC::GFP expressing wild-type and septin mutant embryo, imaged with two-photon microscopy.
Time interval between acquired frames was 2 min and the acquisition length was 90 min (wild-type) and 98 min (mutant). Video is 706 × faster than real-time. (MOV 825 kb)
Top view of a MRLC::GFP expressing wild-type and septin mutant embryo during the slow phase of cellularization.
Images were collected at the FC with spinning disk confocal microscopy. Time interval between acquired frames was 10 s and the acquisition length was 44 min. Video is 149 × faster than real-time. (MOV 11781 kb)
Time-lapse imaging of actin bundle formation in the presence of 0.1 μM fly septins.
Time interval between acquired frames was 0.5 s and the acquisition length was 5 min. Video is 7.5 × faster than real-time. (MOV 8893 kb)
Time-lapse imaging of thermally undulating single actin filaments.
Time interval between acquired frames was 0.5 s and the acquisition length was 1 min. Video is 7.5 × faster than real-time. (MOV 889 kb)
Rights and permissions
About this article
Cite this article
Mavrakis, M., Azou-Gros, Y., Tsai, FC. et al. Septins promote F-actin ring formation by crosslinking actin filaments into curved bundles. Nat Cell Biol 16, 322–334 (2014). https://doi.org/10.1038/ncb2921
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2921
This article is cited by
-
Lysyl hydroxylase LH1 promotes confined migration and metastasis of cancer cells by stabilizing Septin2 to enhance actin network
Molecular Cancer (2023)
-
Anillin propels myosin-independent constriction of actin rings
Nature Communications (2021)
-
Mechanistic insight into bacterial entrapment by septin cage reconstitution
Nature Communications (2021)
-
Biomechanical Regulation of Stem Cell Fate
Current Stem Cell Reports (2021)
-
In silico stress fibre content affects peak strain in cytoplasm and nucleus but not in the membrane for uniaxial substrate stretch
Medical & Biological Engineering & Computing (2021)